Tenured and Tenure-Track Faculty

Dr. Lyle Castle
Interim Dean of College of Science and Engineering Full Professor
Office: PS 120A, Pocatello
208-282-7929
Ph.D. Organic Chemistry, University of South Florida, 1992
Research area: Organic Synthesis, Organometallic Synthesis
Student experience required for research: CHEM 111 & 112 and currently enrolled in CHEM 301/303
Student experience gained from research: Synthetic methods, chromatography, 1D and 2D NMR, photochemistry and theoretical methods.
Ideal preparation for: National companies specializing in agrochemical, pharmaceutical, and petroleum. Local companies such as Simplot, AMI, and INL. Also good for pre-medical, pre-dental and graduate schools in science or other areas.
Research Focus
In 1979 a method for breaking water into hydrogen and oxygen using sunlight was reported in the literature. The water-splitting scheme is shown below:
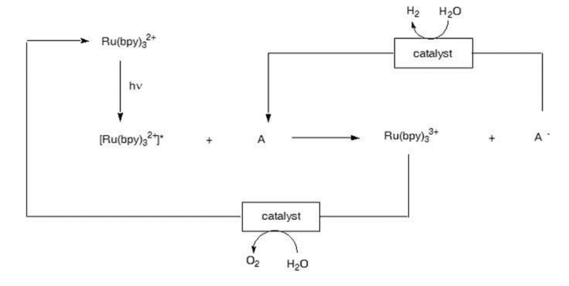
The above scheme shows the potential for generating water through electron transfer processes driven by sunlight. The process shown above does in fact generate hydrogen and oxygen using sunlight. However, the process is so slow that it is not useful. The limiting factor is the lifetime of the initially formed excited state termed the Metal to Ligand Charge Transfer state (MLCT). The shorter the lifetime of this state the less likely the process becomes. Hence, to successfully utilize this approach for solar energy conversion new compounds must be discovered that generate longer MLCT lifetimes.
The research in my laboratory focuses on the synthesis of new heterocyclic compounds containing the 1,10-phenanthrolin moiety for chelation with d-block metals with potential application as photosensitizers for solar energy conversion devices. The aim is to prepare the ligands, their metal complexes and to then measure the wavelength and lifetimes of their MLCT's. Once a variety of such compounds have been prepared and their MLCT's measured the long-term aim would be to begin to develop structure activity relationships where MLCT lifetimes would correlate with specific structural features.
The synthetic scheme currently in place in my laboratory utilizes the photocyclization reaction to construct the 1,10-phenanthroline moiety. The basic synthetic strategy is shown below:
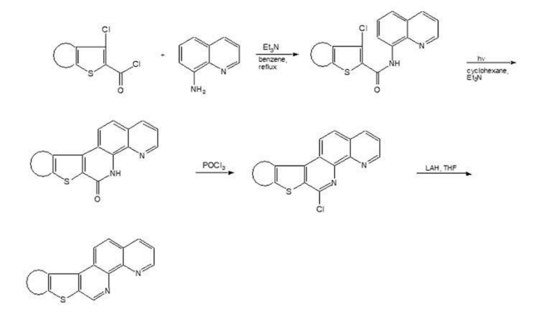
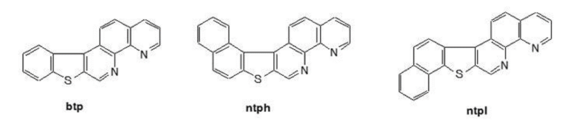
Using this approach, we have synthesized the heterocyclic compounds shown below as well as
their metal complexes: Ru(bpy)2btp+2, Ru(bpy)2ntph+2 and Ru(bpy)2ntpl+2 where bpy refers to
bipyridine.
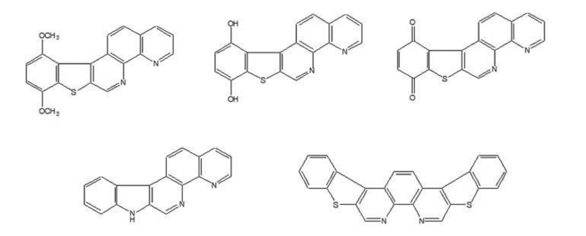
We are currently in the process of synthesizing these related structures through a modification
of the synthetic scheme shown above.
Finally, all the synthesized compounds will be used to prepare metal complexes for
measurement of their MLCT's.
Recent Publications
"The First Synthesis of bis-Fused 1,10-Phenanthrolines via Double Photocyclization of N,N-Disubstituted o-Phenylenediamine," T.A. Elmaaty and L.W. Castle, Synthesis, 9, 1402-1404, 2006.
"A New Methode for the Synthesis of Indeno[1,2-b]thiophene with Subsequent Ring Exspansion to form Substituted Thieno[3,2-c]quinoline," L.W. Castle and T.A. Elmaaty, J. Heterocycl. Chem., 43(3), 629, 2006.
"Facile Regiocontrolled Synthesis of Trialkylsubstituted Pyrazines," T.A. Elmaaty and L.W.Castle, Organic Lett., 7(24), 5529-5530, 2005.
"Chromate Oxidation of a-Nitro Alcohols to a-Nitro Ketones: Significant Improvement to a Classic Method," T.A. Elmaaty and L.W. Castle, Molecules, 10, 1458-1461, 2005.
"Synthesis and Total 1H-NMR Assignment of [1]Benzothieno[2,3c][1,10]phenanthroline," A.P. Halverson, L.W. Castle, J. Heterocyclic Chem., 33, 727, 1996.

Dr. Caryn Evilia
Full Professor
Office: PSC 356, Pocatello
208-282-4373
Ph.D. Biological Chemistry, University of Pennsylvania, 1998
Research area: Biochemistry, Structural Biology, Microbiology, Metagenomics
Student experience required for research: BIOL 1101, 1102, enrolled in 2235/2221; CHEM 1111, 1112, enrolled in CHEM 3301, 3302
Student experience gained from research: Enzyme assays with traditional and non-traditional enzymes, molecular biology, biochemistry and microbiological methods, structural biology instrumentation, bioinformatics, and computer model building.
Ideal preparation for: Professional schools- especially Graduate and Medical school, and the Biotechnology and Pharmacology industries.
Research Focus
My research program focuses on the effects of extreme conditions on microbes, proteins and nucleic acids. In particular, my lab studies the structural adaptations in proteins and the genome, that microbes need to have to survive under these conditions. My lab studies these adaptations through a combination of experimental and computational biochemistry and microbiology, structural biology, and bioinformatics.
Proteins and nucleic acids are very sensitive to their chemical and physical surroundings. Like the organisms from which they are isolated, these molecules tend to function and remain stable over a relatively narrow range of environmental conditions such as temperature, pH, salinity, and pressure.
What those narrow conditions are, however, can vary widely. Most organisms grow best at about 37 oC, under one atmosphere of pressure, with less than 0.1 M salt. But many extremophile microbes do not; some grow in hot springs where the temperatures range from 40 oC to ~100 oC, or in hypersaline environments, such as the Great Salt Lake, where the salinity is over saturation at +250 grams/L total salts. These organisms must have evolved adaptations to maintain the structural integrity of their proteins, nucleic acids, and membranes under these conditions, because non-extremophile proteins rapidly denature and/or lose activity when subjected to them. My lab focuses on adaptations for the extreme halophiles and related organisms, to determine how they survive and thrive in their extreme environment.
Recent Publications
Brininger, C., Spradlin, S., Lori, C., Evilia, C. 2018. “The more adaptive to change, the more likely you are to survive: Protein adaptation in extremophiles”. Seminars in Cell and Developmental Biology, 84, 158-169. DOI: 10.1016/j.semcdb.2017.12.016
Evilia, C. 2018. “Understanding protein adaptations can help us solve real problems”. Seminars in Cell and Developmental Biology, 84, 127-128. DOI: 10.1016/j.semcdb.2018.02.009
S. Spradlin, L. Cobani, C. Brininger, C. Evilia, Book chapter, titled, “Archaea were trailblazers in signaling evolution: Protein adaptation and structural fluidity as a form of intracellular communication”, in Biocommunication in Archaea, Editor: Günther Witzany, Springer International Publishing AG, Cham publishers. DOI: 10.1007/978-3-319-65536-9_12
Hanson, R., Evilia, C., Gilmer, J., Woods, L., Black, B., Flores, R., Pfau, J. C. 2016. “Libby Amphibole-Induced Mesothelial Cell Autoantibodies Bind to Surface Plasminogen and Alter Collagen Matrix Remodeling.” Physiological Reports, 4 (15, e12881), 1-12. DOI: 10.14814/phy2.12881
C. J. Reed, Bushnell, S. C. Evilia. 2014. "Circular dichroism and fluorescence spectroscopy of cysteinyl-tRNA synthetase from Halobacterium salinarum ssp. NRC-1 demonstrates that group I cations are particularly effective in providing structure and stability to this halophilic protein." PLoS ONE, 9 (3), e89452. DOI: 10.1371/journal.pone.0089452. eCollection 2014

Dr. Lisa Goss
Associate Professor
Office: PSC 340A, Pocatello
208-282-4373
Ph.D. Physical Chemistry, University of Colorado at Boulder, 1998
Research Areas: Medical isotopes and radiochemistry, high resolution vibrational spectroscopy and atmospheric chemistry, pedagogy of physical chemistry
Student experience required for research: CHEM 111, CHEM 112, enrolled in CHEM 232 for project 1, enrolled in CHEM 351 for projects 2 & 3, completion of CHEM 112 and programming or SME experience for project 4
Student experience gained from research: Ion exchange separations and radiochemistry for project 1, rotational and vibrational spectroscopy, quantum mechanics for projects 2 & 3, programming for project 4
Ideal preparation for: Graduate school in chemistry, employment in analytical labs, teaching chemistry
Research Focus
1) Ion exchange separations for the preparation of medical isotopes
Project 1 is part of a collaboration with the Idaho Accelerator Center to develop electron linear accelerator sources of medical isotopes. Medical imaging frequently uses technetium-99m which is produced by the decay of molybdenum-99:
99Mo → 99mTc + γ
Molybdenum-99 is currently produced by separation from a mixture of nuclear fission products. The reactors which are used for this process are aging which puts future supply in jeopardy. New methods of producing molybdenum-99 or alternative radionuclides are therefore needed.
The current focus of the product is the production of copper-67 via a photonuclear reaction in zinc:
68Zn + γ → 67Cu + 1p
This isotope decays by β emission with an average particle energy of 141 keV and also emits γ photons of energies 93 keV and 184 keV. These two characteristics make copper-67 suitable for simultaneous imaging and radioimmunotherapy which is a very desirable combination.
Our part of this project is the chemical separation of the copper-67 from the zinc target material. This is accomplished using an anion exchange resin under hydrochloric acid conditions where it is very selective for zinc and a chelating ion exchange resin under conditions where it is selective for copper.
The key criteria for a successful separation are specific activity, purity, and total yield. Specific activity is the ratio of the activity of the target isotope to the total mass of all isotopes of that element, usually in kCi/g. The purity refers to the absence of other elements, in this case primarily zinc. Total yield is the amount of the target isotope produced.
Future work will include developing other separations for radionuclei which are suitable for medical imaging, radioimmunotherapy, or both.
2) Rotationally resolved vibrational spectroscopy of atmospheric pollutants containing methyl rotors
Methyl nitrite is a gas phase molecule formed in the atmosphere by the reaction of methoxy radicals with nitric oxide:
CH3O• + NO• → CH3ONO (1)
In equation (1), the dots indicate that those molecules are radicals with unpaired electrons. This makes these radicals particularly reactive. In daylight, methyl nitrite rapidly photolyzes to reform these radicals:
CH3ONO + hν (λ≤440 nm) → CH3O• + NO• (2)
Since the methoxy radical (CH3O•) and nitric oxide (NO•) are both key intermediates in photochemical air pollution, methyl nitrite serves as a night-time reservoir, or storage molecule, that contributes to the "aged smog" phenomenon. This is where a pollution episode is worse on a second day due to pollutants that have not been removed from the atmosphere overnight.
High resolution Fourier Transform infrared spectrometry has been used on a variety of reactive molecules to measure their abundance in the atmosphere. This measurement technique uses the Beer-Lambert law which says that the absorbance is proportional to the concentration of the absorbing molecule and the path length over which this absorption occurs:
A(λ) = - Ln ( I(λ) / Io(λ) ) = (α(λ))x(concentration)x(pathlength) (3)
Here I(λ) and Io(λ) are light (as a function of wavelength) with and without absorber. The absorption coefficient is a characteristic of the particular molecule being studied. In the atmosphere, the concentrations are typically so low that a long pathlength (100's of meters) is typically required. The absorption coefficient is measured in a separate experiment, the pathlength is determined by the geometry of the experiment, and the absorbance is calculated from measurements of I(λ) and Io(λ) so that the concentration can then be determined. The role of laboratory studies such as in this project is to measure the absorption coefficient as a function of wavelength (λ), understand how it changes with pressure and temperature, and, most importantly, to understand why the absorption coefficient (or the "spectrum") of the molecule looks the way it does. This understanding is particularly crucial when the spectrum of the molecule being measured overlaps with the spectrum of other molecules that are present in the atmosphere and therefore might interfere with determining the concentration of methyl nitrite. Figuring out which lines (features in the spectrum) belong to which molecules can be a challenging task.
In a simple linear molecule like CO2, the spacing between the lines is directly proportional to the moment of inertia. Using the masses of the atoms and the moment of inertia, the bond lengths can be determined. For a large, asymmetric molecule like methyl nitrite, the pattern of lines is more complex and figuring out which lines correspond to which transitions between energy levels ("assigning the spectrum") is an involved process. The amount of information that can be obtained is also larger as well. The methyl group in methyl nitrite can rotate and this internal rotation can be investigated. Methyl nitrite also possesses two isomers. These two isomers only differ by rotation about the O-N bond. Both of these isomers are present in a sample of methyl nitrite and are observed in the spectrum.
The objective of this project is to measure and analyze the vibrational bands of methyl nitrite. The measurements will be made in the William R. Wiley Environmental Molecular Sciences Laboratory (EMSL) which is operated by Pacific Northwest National Lab (PNNL) for the Department of Energy. The High Resolution Infrared Spectroscopy Laboratory within EMSL has a Bruker IFS120 spectrometer that is capable of measuring infrared spectra at 0.0015 cm-1 resolution. Once the spectra have been measured at high resolution, they will be assigned and a nonlinear least squares fit will be performed to extract the constants characterizing the structure, cis-trans isomerism, and internal rotation of the molecule. Additional methyl rotor containing molecules to be investigated include methyl glyoxal, CH3COCHO.
3) Rotationally resolved vibrational spectroscopy of CFC replacement molecules
CFC's have been implicated in polar ozone depletion ("the ozone hole") as the source of chlorine atoms. Replacements for CFC's have focused on molecules which are inert enough to be used for the same purposes as CFC's but reactive enough to break down in the troposphere rather than reaching the stratosphere before breaking down like CFC's. Release of Cl in the decomposition of CFC's in the lower stratosphere results in catalytic destruction of ozone:
Cl + O3 → ClO + O2 (1)
ClO + O → Cl + O2 (2)
sum: O3 + O → 2 O2 (3)
This project will measure and analyze the rotationally resolved spectrum of replacement molecules such as HFC-125 (C2F5H) for reasons similar to those in project 2. CFC replacements could potentially be used in large amounts and are more stable than many pollutants and therefore reach concentrations in the atmosphere greater than that of a pollutant like methyl nitrite. An additional concern with these CFC replacements beyond pollutants like methyl nitrite is their absorption of IR radiation in the atmosphere. Changes in the absorption of IR radiation in the atmosphere (the greenhouse effect) can affect global climate.
4) Development of chemistry pedagogy materials using Mathematica
Project 4 focuses on the development of pedagogical materials for chemistry in general and physical chemistry in particular. The symbolic math engine Mathematica has a built-in command, "Manipulate[ ]", which makes it very straight-forward to turn a static visual into an interactive simulation. The Wolfram Demonstrations Project is a free online resource that publishes these "demos". For examples, see the following:
Lever Rule Applied To Phase Diagram For Partially Miscible Liquids
Rovibronic Infrared Spectrum Of A Rigid Diatomic Rotor
Future work on this project will involve design and production of more of these demos for physical chemistry and other areas of chemistry.
Recent Publications
Lisa M. Goss, “Reflections on the effect of the flipped classroom on students’ difficulties with homework in physical chemistry“, in Teague, C. and Gardner, D. eds, Engaging Students in Physical Chemistry, ACS Symposium Series, American Chemical Society: Washington, DC, 2018.
Lisa M. Goss, "The Use of Active Learning and a Symbolic Math Program in a Flipped Physical Chemistry Course", in Muzyka, J. and Luker, C. eds.; The Flipped Classroom Volume 1: Background and Challenges ACS Symposium Series; American Chemical Society: Washington, DC, 2016.
"The high-resolution, jet-cooled infrared spectrum of pentafluoroethane", L.M. Goss, W.R. Hess, T.A. Blake, R.L. Sams, Journal of Molecular Spectroscopy, 265, 8185, 2011.
"Rotationally Resolved Spectroscopy of the ν8 Band of cis-Methyl Nitrite," L.M. Goss, C.D. Mortensen, T.A. Blake, Journal of Molecular Spectroscopy, 225(2), 182-188, 2004.
"A Demonstration of Acid Rain and Lake Acidification: Wet Deposition of Sulfur Dioxide," L.M. Goss, Journal of Chemical Education, 80, 39-40, 2003.

Dr. Andrew Holland
Full Professor
Office: PSC 352, Pocatello
208-282-4373
Postdoctoral Studies, Heterogeneous Catalysis, University of California, Berkeley, 2002-2004
Ph.D. Organometallic Chemistry, University of California, Berkeley, 2002
Research area: Organometallic Chemistry, Inorganic Chemistry
Student experience required for research: CHEM 111, CHEM 112, enrolled in CHEM 301
Student experience gained from research: Synthetic methods, mechanistic methods, air-sensitive techniques, NMR.
Ideal preparation for: Graduate school in chemistry; petrochemical, specialty, and pharmaceutical chemical industries.
Research Focus
1) Basic Late Metal Complexes in Catalysis
Anionic heteroatom ligands on electron-rich late metal centers may serve both basic and nucleophilic functions, but are frequently too high in relative energy to be regenerated cleanly in catalytic systems. We are interested in exploring, from a mechanistic inorganic perspective, scenarios that might overcome that problem to enable new bond activation and formation reactivity. Specifically, we aim to use chelating nitrogen ligands to produce strongly basic catalysts that can heterolytically activate H-X substrates to yield nucleophilic M-X species. We explore this idea through the preparation and study of new transition metal complexes.
2) Microwave Heating in Organometallic Chemistry
Microwave heating has proved to be a powerful tool in the synthesis of ligands, and we are currently exploring the potential and origins of the apparent acceleration associated with the technique. This work includes both practical application of microwaves to the syntheses of ligands and their complexes, and investigation of the kinetic changes (or lack thereof) induced by microwave heating.
3) New Approaches to Inorganic Materials Synthesis
The synthesis of inorganic materials has historically been approached largely from an entirely empirical perspective, but rational synthesis using well-defined solution phase techniques and molecular precursors has shown promise as an approach to targeting specific surface structures, particle sizes, and other properties. We are currently exploring the preparation of novel molecular precursors for nanoparticles with photovoltaic applications.
Recent Publications
"Synthesis and Reactivity of Sterically-Hindered Amido-Phosphine Complexes of Rhodium and Iridium." K. R. Seipel, K. L. Myers, M. Nguyen, B. B. Twamley, A. W. Holland, Inorg. Chem., manuscript in preparation.
"Kinetics of Reductive Elimination from Pt(IV) as a Probe for Non-Thermal Microwave Effects." C. Lombard, K. L. Myers, Z. H. Platt, A. W. Holland. Organometallics, submitted.
"Microwave-Assisted Synthesis of Phenylene-Bridged Amino-Phosphine Ligands: Acceleration of N-Arylation and Aryl Fluoride Phosphorylation Reactions." K. R. Seipel, Z. H. Platt, M. Nguyen, A. W. Holland. J. Org. Chem.73, 4291-4294, 2008.
"New Fe/SiO2 Materials Prepared Using Molecular Precursors: Synthesis, Characterization, and Catalysis." A. W. Holland, G. T. Li, Ahmed M. Shahin, G J. Long, A. T. Bell, T. D. Tilley. J. Catal. 235-1, 150-163, 2005.
"Effects of Phosphine Chelation on the Reactivity of Monomeric Parent Amido Complexes: Synthesis and Reactivity of Such a Complex Bearing Monodentate Ligands," A.W. Holland, R.G. Bergman, J. Am. Chem. Soc.124, 14684-14695, 2002.

Dr. Courtney "Cori" Jenkins
Associate Professor
Office: PSC 347, Pocatello
208-282-4373
Postdoctoral Studies: Chemical Biology, California Institute of Technology, 2015-2016
Ph.D. Chemistry, Purdue University, 2015
Bachelor of Science Biochemistry, Saint Louis University, 2010
Research area: Sulfur-based Polymer Chemistry
Student experience required for research: CHEM 1111, CHEM 1112
Student experience gained from research: Polymer synthesis and characterization, materials analysis.
Ideal preparation for: Employment in a wide-range of chemical industries, teaching, and preparation for graduate school or professional schools.
Research Focus
Elemental sulfur (S8) is produced in abundance during petroleum refinement, generating millions of tons of waste. The Jenkins group uses a technique called inverse vulcanization to make new polymeric materials from elemental sulfur. Sulfur becomes the backbone and acts as the solvent in this reaction eliminating the need for volatile organic solvents. Natural monomers including those from garlic have been incorporated into these materials further promoting aspects of green chemistry.
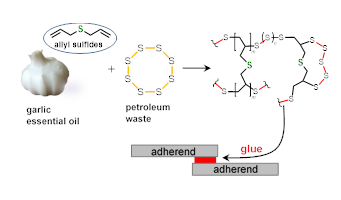
In the lab, students examine fundamental aspects of polymer synthesis and characterization. These polysulfides contain dynamic bonds, which offers an opportunity to create modifiable polymer frameworks. These materials have applications in heavy metal remediation and adhesives among others. The ultimate goal in the lab is to utilize petroleum waste products for the development of functional materials.

Students will learn polymer synthesis and a variety of instrumentation techniques including NMR, ATR-IR, UV-Vis spectroscopy, gel permeation chromatography (GPC), and differential scanning calorimetry (DSC) as well as common chemistry software. Additionally, regular group meetings will provide both written and verbal communication skills.
Recent Publications
Sayer, K. B.; Miller, V. L.; Merrill, Z.; Davis, A. E.; Jenkins, C. L. Allyl Sulfides in Garlic Oil Initiate the Formation of Renewable Adhesives. Polymer Chemistry. 2023. 14(26), 3091-3098.
Davis, A. E.; Sayer, K. B.; Jenkins, C. L. A Comparison of Adhesive Polysulfides Initiated by Garlic Essential Oil and Elemental Sulfur to Create Recyclable Adhesives. Polymer Chemistry. Accepted. https://doi.org/10.1039/D2PY00418F
Eder, M. L.; Call, C. B.; Jenkins, C. L. Utilizing Reclaimed Petroleum Waste to Synthesize Water-soluble Polysulfides for Selective Heavy Metal Binding and Detection. ACS Applied Polymer Materials. 2022. 4(2), 1110–1116. https://doi.org/10.1021/acsapm.1c01536.
Orme, Kennalee; Fistrovich, Alessandra H.; Jenkins, C. L. Tailoring Polysulfide Properties through Variations of Inverse Vulcanization.” Macromolecules. 2020. 53, 9353-9361.
Herrera, C.; Ysinga, K. J. ; Jenkins, C. L. “Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives.” ACS Applied Materials and Interfaces. 2019. 11(38), 35312-35318.

Dr. John Kalivas
Full Professor
Office: PSC 253A, Pocatello
208-282-4373
Ph.D. Analytical Chemistry, University of Washington, 1982
Research areas: Analytical Chemistry, Chemometrics, Chemical Education
Student experience required for research: CHEM 1111 and CHEM 1112
Student experience gained from research: Data analysis, modeling, computational chemistry, spectroscopy, teaching
Ideal preparation for: Chemical industry, pharmaceuticals, medical, environmental, and agriculture research, teaching, and preparation for graduate school in Chemistry or other professional schools.
Research Focus
Our analytical chemistry research focus is in an area termed chemometrics concerned with mathematical and statistical analysis of chemical data. While this are of research was well established in the early 1970’s, the area is now popularized as data science, machine learning, and artificial intelligence.
We concentrate on developing computer algorithms to determine mathematical relationships between chemical data and analyte properties desired such as the concentration estimate of a substance in a sample. Algorithms are trained to recognize data patterns and predict properties for new situations. For example, training an algorithm to distinguish noninvasively measured spectra of cancer cells from noncancer cells. Our mission is creating new algorithms to solve difficult analytical chemistry problems by leveraging sample and measurement matrix effects (hidden physicochemical properties) as information to work with. Some research projects follow.
Multivariate Calibration (Modeling): Central to many disciplines is multivariate calibration including food analysis, food adulteration detection and authentication of product origin, environmental monitoring, industrial process control, medical diagnosis such as disease detection, pharmaceutical analysis, forensic analysis, detection of hidden radioactive material, and the list goes on. Work in our laboratory consists of developing new mathematical processes and the corresponding computer algorithm implementations in order to improve calibration quality in conjunction with eliminating user decisions making calibration and subsequent analyte predictions automatic. A persistent multidisciplinary problem is developing a calibration model in one set of environmental, instrumental, physicochemical conditions (the primary source conditions) to now work in new target conditions, i.e., the calibration maintenance (transfer) problem.
Our laboratory has two approaches to solve this problem that has been restricting onsite analyses with handheld devices including consumer applications. One solution is local modeling.
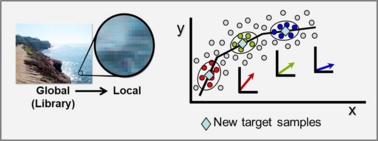
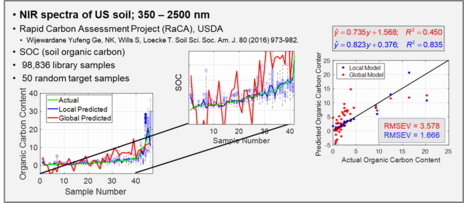
With the ever-growing availability of spectral data, e.g., near infrared (NIR), Raman, fluorescence, and other spectral processes, there is a high need for computer algorithms to mine through such data libraries and identify those spectra similar to a spectrum just measured for a new target sample. The user is interested in using the new spectrum for quantitative analysis of an analyte, perhaps glucose content for diabetic, and a model must first be formed using calibration spectra matrix matched to the new target sample spectrum. Because the analyte amount in the new sample is not known, identifying fully matched calibration samples from a library is not a simple task. However, we have developed an algorithm to accomplish this objective that we call local adaptive fusion regression (LAFR). Key takeaways for LAFR are: (1) models are formed by an understandable process based on physicochemical properties and principles with a Beer’s law-like relationship, (2) final calibration sets bracket target sample analyte values, and (3) all adjustable parameters are self-optimized. Ongoing work involves providing a reliability measure based on the probability of a correct prediction.
The second approach is model updating by the transfer learning approach domain adaptation. In collaboration with Erik Andries at Central New Mexico Community College, we now have a null augmented regression (NAR) algorithm that correctly predicts new target samples. Models are formed using the new unlabeled target samples for which is prediction is desired.
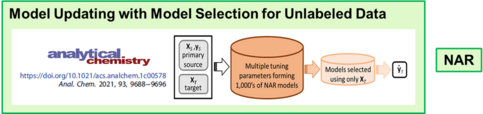
The limitation to advancing model updating for practical use has been model selection due to the multiple tuning parameters requiring optimization. We recently developed model diversity prediction similarity (MDPS), an algorithm that selects accurate models for the unlabeled samples. Up to three tuning parameters can be involved and the approach is generalizable to additional tuning parameters.
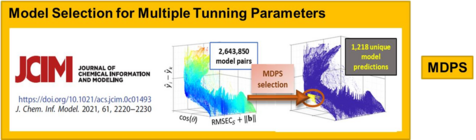
Both LAFR and NAR/MDPS can make automatic analysis by handheld devices easier, i.e., bringing the lab to the sample.
Data Set Characterization, Outlier Detection, and Classification: Key to both local modeling and model updating is assessing the similarity of a primary source calibration set to new unlabeled target samples. Our laboratory is further developing two measures termed indicators of system uniqueness (ISU). One characterizes hidden physicochemical spectral differences using hundreds of spectral similarities measures (ISUX) and the other is based on analyte amount similarities (ISUy) even though the new target samples are unlabeled. Ongoing work involves combining the two ISU values for a membership product value (ISUXy)
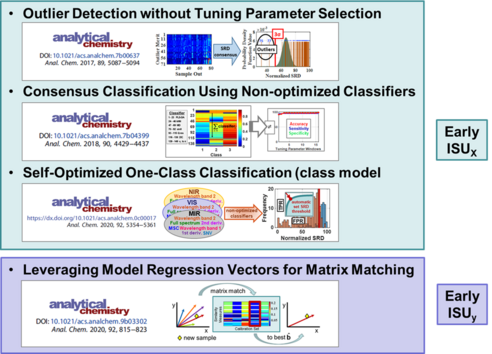
Education: We also have on going chemical education projects in our research laboratory. These consist of developing new laboratory exercises for quantitative analysis and instrumental analysis. In the past, our laboratory has developed new guided inquiry labs for general chemistry and quantitative analysis including chemical analysis of live trout for their fat and moisture contents. Currently, we are working on new labs for instrumental analysis that provide greener approaches to multivariate calibration using one of our newly developed NAR model updating process.
Service-learning is becoming ever more important in the education of students to become responsible chemists. Service-learning involves students in thoughtfully organized service activities addressing community needs and complementing students’ academic studies. Service-learning results from a curriculum that extends the classroom into the community combing education and service and includes class time to reflect on the service experience. Our research desires are to develop new service-learning components in analytical chemistry courses
Recent Publications
C. Spiers, J.H. Kalivas: “Calibration Model Updating to Novel Sample and Measurement Conditions without Reference Values”, Analytical Chemistry. In press (2021). https://doi.org/10.1021/acs.analchem.1c00578
C. Spiers, J.H. Kalivas: “Reliable Model Selection without Reference Values by Utilizing Model Diversity with Prediction Similarity”, Journal of Chemical Information and Modeling, 61, 2220-2230 (2021). https://doi.org/10.1021/acs.jcim.0c01493
Struhs, S. Hansen, A. Mirkouei, M.M. Ramirez-Corredores, K. Sharma, R. Spiers, J.H. Kalivas: “Ultrasonic-Assisted Catalytic Transfer Hydrogenation for Upgrading Pyrolysis-oil”, Ultrasonics Sonochemistry, 73, 105502 (2021), Open Access https://doi.org/10.1016/j.ultsonch.2021.105502
H. Kalivas, S.D. Brown: "Calibration Methodologies" in Comprehensive Chemometrics: Chemical and Biochemical Data Analysis, 2nd Edition, editors-in-chief S. Brown, R. Tauler, and B. Walczak, Elsevier, The Netherlands, (2020) https://doi.org/10.1016/B978-0-12-409547-2.14666-9
K. Chabuka, J.H. Kalivas: “Application of a Hybrid Fusion Classification Process for Identification of Microplastics Based on FTIR Spectroscopy”, Applied Spectroscopy, 74, 11671183 (2020) https://doi.org/10.1177/0003702820923993.
MATLAB Code
For MATLAB code to preform Tikhonov regularization without reference samples, see MATLAB Code
For MATLAB code to preform sum of ranking differences (SRD), see 2013_12_16_SRD.7z
For MATLAB code to preform fusion classification, see 2018_3_1_ClassificationCode.7z
For MATLAB code to preform Model updating by sample and feature augmentation, see 2018_9_13_SAFA
For MATLAB code to preform single-class fusion classification with SRD, see 2019_11_25_SingleClassCode.7z
For MATLAB code to preform model selection by model diversity and prediction similarity, see 2020_7_1_MDPS.7z
For MATLAB code to perform physicochemical responsive integrated similarity measure (PRISM) with SRD, see PRISM_SRD.7z
For MATLAB code to perform null augmented regression constant analyte (NARCA), see NARCA.7z
.jpg)
Dr. Leslie Nickerson
Assistant Professor
Office: PSC 361, Pocatello
208-282-4373
Postdoctoral Studies: Organic Synthesis and Teaching, Smith College, 2019-2021
Ph.D. Chemistry, University of California, Davis, 2019
Bachelor of Science Chemistry, University of Idaho, 2014
Research area: Organic methodology and heterogeneous catalysis
Student experience required for research: CHEM 1111, CHEM 1112
Student experience gained from research: Organic synthetic technique, experimental design, heterogeneous catalysis, sustainable chemistry principles, data analysis
Ideal preparation for: Employment in a wide-range of chemical industries particularly with an emphasis on sustainable chemistry, teaching, and preparation for graduate school or professional schools.
Research Focus:
Research in the Nickerson lab is focused on adapting traditional organic transformations that use metal-halide based Lewis acids to more sustainable processes using Lewis acidic heterogeneous materials such as clays and zeolites. This adjustment makes methods safer and more sustainable because the heterogeneous materials are easily recycled and reused and the waste production is far less than when using traditional Lewis acids. Current projects are focused on modifying the Friedel-Crafts acylation and the Prins-pinacol rearrangement.
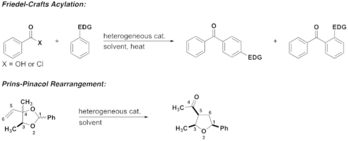
The proposed research will provide student researchers valuable skills in organic synthesis, method development and materials chemistry. Students will learn organic chemistry techniques, experimental design with an emphasis on safety considerations, data analysis, and spectroscopic techniques including NMR and GC. This work will be ideal for any students that want to learn more about implementing sustainable methods in organic chemistry.
Recent Publications
“First-Semester Organic Chemistry During COVID-19: Prioritizing Group Work, Flexibility, and Student Engagement.” L. A. Nickerson, K. M. Shea, J. Chem. Ed. 2020, 97, 3201-3205.
“Transition Metal Catalyzed Insertion Reactions with Donor/Donor Carbenes.” B. D. Bergstroma, L. A. Nickersona, J. T. Shawa, L. W. Souzaa, Angew. Chem. Int. Ed. 2020, 10.1002/anie.202007001. aAuthors are listed alphabetically by last name. “Enantioselective Synthesis of Isochromans and Tetrahydroisoquinolines by C–H Insertion of Donor/Donor Carbenes.” L. A. Nickerson, B. D. Bergstrom, M. Gao, Y-S. Shiue, C. J. Laconsay, M. R. Culberson, W. A. Knauss, J. C. Fettinger, D. J. Tantillo, J. T. Shaw. Chem. Sci. 2020, 11, 494-498.
“Au(I)-Catalyzed Synthesis of Trisubstituted Indolizines from 2-Propargyloxypyridines and Methyl Ketones.” M. D. Rossler, C. T. Hartgerink, E. E. Zerull, B. L. Boss, A. K. Frndak, M. M. Mason, L. A. Nickerson, E. O. Romero, J. E. Van de Burg, R. J. Staples, C. E. Anderson. Org. Lett. 2019, 21, 5591-5595.
“Enantioselective Synthesis of Indolines, Benzodihydrothiophenes, and Indanes by C–H Insertion of Donor/Donor Carbenes.” L. W. Souza,† R. A. Squitieri,† C. A. Dimirjian, B. M. Hodur, L. A. Nickerson, C. N. Penrod, J. Cordova, J. C. Fettinger, J. T. Shaw. Angew. Chem. Int. Ed. 2018, 130, 15433-15436. †These authors contributed equally.

Dr. Joshua Pak
Department Chair - Full Professor
Office: PSC 145, Pocatello / PSC 360, Pocatello
208-282-4373
Ph.D. Chemistry, University of Oregon, 1999
Master of Science Chemistry, Duquesne University, 1995
Bachelor of Science Chemistry and English Literature, Whittier College, 1993
Research area: Organic, Organometallic, Materials and Polymer Chemistry
Student experience required for research: First, second and third year chemistry majors or Enrolled in General Chemistry or Organic Chemistry
Student experience gained from research: Organic and organometallic synthetic techniques, NMR, air-sensitive techniques, computational chemistry, mass spectrometry
Ideal preparation for: Polymers, materials, pharmaceuticals, medical research, preparation for graduate school in chemistry and for professional school such as medical, dental and pharmacy schools.
Research Focus
Research in Pak Lab consists of synthesis and study of “non-natural” nano- to meso-scale materials. We utilize modern synthetic methods for the preparation of novel inorganic, organic, organometallic, and organic-inorganic hybrid materials with technologically important properties such as (semi-)conductivity and nonlinear optical (NLO) activities. In addition to preparation of organic, organometallic, and inorganic materials, we collaborate with various research groups here at ISU and around the world in analysis, device construction, and device testing.
Recent Publications
“Multi-step synthesis of hexaarylbenene for organic chemistry lab” Lamm, Ashley N.; Puhl, Dillon; Cox, Abbigail; Pak, Joshua J., Chemical Educator, 2019, 24, 17-173.
“Synthesis and Characterization of Bimetallic Single-Source Precursors (Ph3P)2M(µ-SEt)2E(SEt)2 for MES2 Chalcopyrite Materials (M = Cu, Ag and E = In, Ga, Al)” Kelsey R. Margulieux, Chivin Sun, Matthew T. Kihara, Adam C. Colson, Lev N. Zakharov, Kenton H. Whitmire, Andrew W. Holland, and Joshua J. Pak, European Journal of Inorganic Chemistry, 2017, 2068–2077, DOI: 10.1002/ejic.201700115.
“A Multicomponent Metal-Organic Framework with a High Tolerance for Vacancy Defects” Lee, Seok; Doussot, Celine; Baux, Anthony; Liu, Lujia; Jameson, Geoffrey; Richardson, Christopher; Pak, Joshua J.; Trousselet, Fabien; Coudert, François-Xavier; Telfer, Shane, Chem. Mater. 2016, 28(1), 368–375, DOI: 10.1021/acs.chemmater.5b04306.
“Fabrication and Characterization of Thin Film Solar Cell Made From CuIn0.75Ga0.25S2 Wurtzite Nanoparticles” Fengyan Zhang, Chivin Sun, Cyril Bajracharya, Rene G. Rodriguez, Joshua J. Pak, Journal of Nanomaterials, 2013, Article ID 320375, 5 pages, 2013. doi:10.1155/2013/320375.
“A Large-Scale Synthesis and Characterization of Quaternary CuInxGa1-xS2 Chalcopyrite Nanoparticles via Microwave Batch Reactions” Chivin Sun, Richard D. Westover, Gary Long, Cyril Bajracharya, Jerry Harris, Alex Punnoose, Rene G. Rodriguez, and Joshua J. Pak*, Int. J. Chem. Eng. 2011, Article ID 545234.

Dr. René Rodriguez
Associate Dean for College of Science and Engineering - Full Professor
Office: PSC 120B, Pocatello / PSC 343, Pocatello
208-282-4373
Ph.D. Physical Chemistry, University of Idaho, 1987
Master of Science Physical Chemistry, University of Minnesota, 1984
Bachelor of Science Chemical Engineering, University of Colorado, 1981
Research area: Plasma Enhanced Thin Film Deposition of Photovoltaic Materials, Spectroscopic, Chromatographic, and Spectrometric Analytical Instrumentation, Vibrational Spectroscopy
Student experience required for research: CHEM 1112
Student experience gained from research: Instrumentation Design, electronics, spectroscopy, mass spectrometry, Thin film deposition techniques for Semiconductor Materials
Ideal preparation for: Chemical industry, computer chip manufacturing, analytical laboratory, preparation for graduate school in Physical/Analytical Chemistry or for Engineering School
Research Focus
1) Plasma Enhanced Chemical Vapor Deposition of Thin Films of Semiconductors
We are developing PECVD methodology to deposit amorphous chalcogenide materials, like GexSy, GexSey, as possible phase memory or ion-conductive memory materials. Germanium sulfide is also known to have a large difference in conductivity between it amorphous phase and its crystalline phase. This is essential for phase memory devices. It is also known to have a high ionic conductivity toward Ag and lithium ions. This property is essential for ion conductive or bridge-conductive memory (also known as resistive RAM) and in Li battery applications. GexSey is likely to have higher ion conductivity and we are investigating the ion-conductivity as a function of the relative ratio of the Ge to Se in the PECVD films. We are also doping more metallic elements like Sn into the films and are studying how this affects the conductivity. This work is being performed in conjunction with Prof. Kris Campbell at Boise State University.
It is apparent at this point in time that we have reached a junction in the path we will take to power the world of the future. If we intend to have sufficient non polluting energy for the growing and maturing world it is apparent that solar energy conversion will likely represent a large component of the total energy sources available for humankind. Thin films of germanium and tin sulfides and selenides also have potential application in this area.
2) Investigation of Intermolecular Interactions in Ionic Liquids by Density Functional Theory Computations, Physical Measurements, and Spectroscopic Investigation
Ionic Liquids are ionic compounds that are liquids at room temperature. Many of these liquids consist of cation with long organic groups and a central positively charged atom, and an anion like dicyanamide or bistriflimide. We are working with Dr. Donna Baek’s group at the INL performing fundamental studies of several of these liquids, which potentially could be used as electrochemical media for rare-earth metal recovery. The viscosity and conductivity of these ionic liquids are important factors that must be considered. We are making conductivity and viscosity measurements on these liquids, at several temperatures, to help gauge strength of the intermolecular interactions present in these liquids. Additionally, 2d NMR studies we are performing suggest that ordering on a nano-sized scale may exist in these liquids. We are also using the computational chemistry program Gaussian 16, to perform Density Functional Theory calculations of the interaction energies to correlate with the physical and spectroscopic measurements. These are being performed via the HPC (High Performance Computing) center at the Idaho National Lab.
3)Thermal Imaging for Serial Number Recovery
We are working on a way to recover defaced serial numbers with a nondestructive method. Currently serial numbers on products like firearms or automobile parts which have been partially or completely filed off are recovered using a wet chemical method. The wet chemical method often recovers the serial numbers piecemeal while etching away the remnant memory of the number that exists in the plastic deformation zone. The plastic deformation zone is an area where the properties of the material have been altered due to the large force needed to imprint the numbers in the material. With the wet chemical method, one may get only one chance at recovering the number, since the remnant of the number is often destroyed as the metal is etched away, and so other non-destructive methods are sought that leave the evidence in a state that is available for further testing or verification at a later time.
We plan to make use of the difference in heat flow in the plastic deformation zone to recover the serial numbers. This will be accomplished through the use of real-time infrared thermography and image processing techniques. Since this technique does not etch away more of the material, it is nondestructive and leaves the sample available for further testing at a later time.
Recent Publication
Rodriguez, R., Baek, D., Case, M. Fox, R. ”Studies Toward the Use of Ionic Liquids and Supercritical CO2 for the Recovery and Separation of Praseodymium from Waste Streams,” Catalysts (2022) 12, 335. https://doi.org/10.3390/catal12030335
Rodriguez, R.G.; Baek, D. L.; Orme, K.; Case, M.E,; Fox, R.V. “Electrochemical, thermodynamic, and physical properties of tetradecyltrihexylphosphonium ([P6,6, 6,14]+) and methyl-propyl piperidinium containing ionic liquids and their propylene carbonate solutions”. Journal of Molecular Liquids (2022) 352, 118607
De Jesus, K., Rodriguez, R., Baek, D., Fox, R., Pashikanti, S., Sharma, K. Extraction of lanthanides and actinides present in spent nuclear fuel partitioning and in electronic waste. J. of Molecular Liquids, Volume 336, 15 August 2021, 116006
“Restoration of defaced serial numbers using lock-in infrared thermography (Part I)”, Journal of Spectral Imaging, (2019) 8, Ikwulono Unobe, Lisa Lau, John Kalivas, Rene Rodriguez and Andrew Sorensen
“Restoration of defaced serial numbers using lock-in infrared thermography (Part II)”, Journal of Spectral Imaging, (2019) 8, Ikwulono Unobe, Lisa Lau, John Kalivas, Rene Rodriguez and Andrew Sorensen

Dr. Jeffrey Rosentreter
Full Professor
Office: PSC 150C, Pocatello
208-282-4373
Ph.D. Environmental/Analytical Chemistry, Colorado State University, 1990
Student experience required for research: CHEM 232 required, CHEM 331-332 recommended
Student experience gained from research: Hands-on experience with a wide array of chemical instrumentation, Introduction to Environmental analysis using standard analytical methods.
Ideal preparation for: Getting a job, 1.3% of the American gross national product is spent on chemical analysis, performed by Analytical Chemists.
Research Focus
1) Archaeological evaluation of prehistoric stone-tools:
Understanding the specific use and purpose of archaeological specimens such as stone axes and grinding stones continues to be a perplexing problem. Traditional form-function relationships provide strong clues to the specific use of these implements. Yet, chemical analysis of residues preserved on these tools may provide key information in identifying how the tool was implemented. Our research goal is to provide chemical analysis capable of identify trace components associated with plant and animal materials. Since different plants and animals produce different types and quantities of organic compounds we can associate these to the toolUs actual use.
2) Geo-thermometry in the Yellowstone Basin:
Underlying the beauty of the Yellowstone plateau and its unique geothermal features is a somewhat restless volcanic caldera. There is much interest in monitoring the activity of this underlying heat source. One measurement of the magma activity is obtained by water chemistry of the geothermal springs located in the greater Yellowstone ecosystem. While it is difficult or impossible to monitor the many individual features, our research proposes a survey measurement be obtained by monitoring chemical indicators from hot springs as they exit the drainage basin in the surface water of three rivers. Research studies include both field and laboratory studies.
3) Solar Remediation of Environmental Contaminants:
A) Photo-Oxidation of inorganic cyanides by solar irradiation.
Cyanide is used in the mining of precious metals, especially gold extraction. The use of solar irradiation is being investigated as a source of decomposition and eventual oxidization of cyanide compounds. The use of semi-conductor oxides as photosensitizers has increased the quantum efficiencies of these processes.
B) Photo-Chemical remediation of TCE using ceramic micro spheres.
Industrialization has lead to the wide use and abuse of toxic organic solvents. The use of TiO2 as a photo-oxidation catalyst for these compounds is well recognized. In an effort to remediate contaminated surface waters in situ, photo-sensitizers are being placed on ceramic micro sphere. These hollow micro spheres allow catalysts to be easily delivered and retrieved from natural waters. The feasibility and effectiveness of using these remediation technologies are being evaluated.
Recent Publications
Rosentreter, J. J.; Hymas, P. (2019). In Hugh Cartwright, Oxford University (Ed.), Using Natural Flowers as Colorful Sulfur Dioxide Detectors (1430-5000 ed., pp. 4). Meridian, ID 83642: The Chem Educator - Journal. http://chemeducator.org
Rosentreter, J. J. (2019). Examination of Chemistry in Everyday Phenomena (pp. 142). Laboratory Manual, Open Education Resource: openaccess.com
Rosentreter, J. J.; Evilia C.M.. (In preparation). Characterization of the Great Salt Lake Hypersaline Environment and Microorganisms. PLOS ONE / Public Library of Science Washington D.C.
Rosentreter, J. J.; Alqurashi, M.A.; Kirkham, M.; Hymas, P. (In preparation). Cyanide Detection in Blood using Indirect Chemical and Photo-Chemical Laboratory Methodologies. Microchemical Journal/Elsevier.
Rosentreter, J. J.; Malamakal, J.; Barnes, K.; Alexander, M. (2017). Solvent selection for fatty acid residue analysis of archeological artifacts. Sample Preparation, 3:1-10pp. https://www.degruyter.com/view/j/sampre
Patents
Rosentreter, J.J.; et al. Continuous Real-Time Measurement of Aquesous Cyanide. Patent No.: US 7,186,379 B2, Patent Date: Mar. 6, 2007. Prior publication data Oct. 17, 2002. Assignee Battelle Energy Alliance, LLC. Contract United States Government, Contract No. DE-AC07-99ID13727.
Rosentreter, J.J.; Gering K.L. Prototype Treatment System for the Destruction of Aqueous Cyanide at Remote Locations. Sponsors: Massachusetts Institute of Technology & Lockheed Martin Idaho Technologies Company (Application under Review).
Teaching Faculty

Necoline "Lena" Allen
Assistant Lecturer
Office: TAB 175A, Idaho Falls
208-282-4373
Master of Science Chemistry, Idaho State University, 2020
Bachelor of Science Chemistry, Idaho State University, 2019
Lena has been a Bengal since 2014. She has worked as a tutor, a teaching assistant, and an adjunct lecturer while here at ISU. She is currently working toward her PhD in Engineering and Applied Science focus on Chemistry. Her current research project is developing a polymeric calibration material for use in photon activation analysis.
.jpg)
Andrew Benard
Assistant Lecturer
Office: PSC 243, Pocatello
208-282-4373
Master of Science Chemistry, UC Davis, 2018
Bachelor of Science Chemistry, University of Idaho, 2014
Recent Publications
“Ambipolar Topological Insulator and High Carrier Mobility in Solution Grown Ultrathin Nanoplates of Sb-Doped Bi2Se3” Zheng Ju, Yasen Hou, Andrew Bernard, Valentin Taufour, Dong Yu, and Susan M. Kauzlarich. ACS Applied Electronic Materials 2019 1 (9), 1917-1923 DOI: 10.1021/acsaelm.9b00415
“Solvent Effects on Growth, Crystallinity, and Surface Bonding of Ge Nanoparticles” Andrew Bernard, Keye Zhang, Daniel Larson, Katayoon Tabatabaei, and Susan M. Kauzlarich. Inorganic Chemistry 2018 57 (9), 5299-5306 DOI: 10.1021/acs.inorgchem.8b00334
“Temperature profile around a basaltic sill intruded into wet sediments” Leslie L. Baker, Andrew Bernard, William C. Rember, Moses Milazzo, Colin Dundas, Oleg Abramov, Laszlo Keszthelyi. Journal of Volcanology and Geothermal Research 2015, 302, 81-86
DOI: 10.1016/j.jvolgeores.2015.06.012.

Carolina Gonzalez-Aller
Adjunct Instructor
Office: PSC 145, Pocatello
(208) 282-4373
Master of Science Biochemistry, University of Colorado Health Sciences Center, 1991
Bachelor of Science Chemistry, University of Colorado
Recent Publications
Leavey PJ, Thurman G, González-Aller C, Zerbe G, Ambruso DR: The effects of stem cell factor and granulocyte colony stimulating factor therapy on the activity of the neutrophil NADPH oxidase enzyme system. J Investig Med, 1998, 46(4):121-126.
Wei LL, González-Aller C, Wood WM, Miller LA, and Horwitz KB. 5'Heterogeneity in human progesterone receptor transcripts predicts a new aminoterminal truncated "C"-receptor and unique A-receptor messages. Mol Endocrinol 4:1833-1840, 1990.
Graham M, Bunn PA, Jewett PB, González-Aller C, and Horwitz KB. Simultaneous measurement of progesterone receptors and DNA indices by flow cytometry: Characterization of an assay in breast cancer cell lines. Cancer Res 49:3934-3942, 1989.
Horwitz KB, Pike AW, González-Aller C, and Fennessey PV. Progesterone metabolism in T47Dco human breast cancer cells. II. Intracellular metabolic path of progesterone and synthetic progestins. J Ster Biochem 25(6):911-916, 1986.
Fennessey PV, Pike AW, González-Aller C, and Horwitz KB. Progesterone metabolism in T47Dco human breast cancer cells. I. 5a Pregnan-3b, 6a-Diol-20one is the secreted produced. J Ster Biochem 25(5A):641- 648, 1986.
Abstracts
Ambruso DR, González-Aller C, Thurman G, Peterson V: Neutrophils (PMNs) from patients with thermal injury have diminished levels of message for p47-phox. J Invest Med, 1995, 43:287a. (Presented at the American Federation for Clinical Research Meeting, San Diego, CA, May, 1995).
Leavey P, Thurman G, González-Aller C, Shpall EJ, and Ambruso D. Neutrophil (PMN) oxidase activity during cytokine therapy. Presented at the American Federation of Clinical Research Meetings, April 29 - May 2, 1994.
Leavey P, Thurman GW, González-Aller C, Shpall EJ, Ambruso DR: NADPH oxidase activity in neutrophil (PMN) subcellular components during cytokine therapy. Clin Res, 1994, 42:304a. (Presented at the American Federation for Clinical Research Meeting, Baltimore, MD, April, 1994).
Leavey P, Thurman GW, González-Aller C, Shpall EJ, Ambruso DR: Neutrophil (PMN) oxidase activity during cytokine therapy. Clin Res, 1994, 42:304a. (Presented at the American Federation for Clinical Research Meeting, Baltimore, MD, April, 1994).
Wei LL, González-Aller C, and Horwitz KB. Oligonucleotide mapping of the multiple human progesterone receptor messages. Presented at the National Endocrine Society Meetings, June, 1990.
Presentations
González-Aller C, Thurman G, Peterson V, Ambruso DR: Neutrophils from patients with thermal injury have diminished levels of message for the oxidase component, p47-phox. Presented abstract at the American Federation for Clinical Research Meeting, San Diego, CA, May, 1995.

Dr. Kate McMurtrey
Assistant Lecturer
Office: TAB 285, Idaho Falls
Ph.D. Chemistry, University of Michigan, 2014
Bachelor of Arts Chemistry, Bryn Mawr College, 2009
Recent Publications
McMurtrey, K. B.; Sanford, M. S. "C F Bond-Forming Reactions," In Cross-Coupling and Heck-Type Reactions from the Science of Synthesis Reference Library. Vol. 2, John P. Wolfe, Ed., Thieme, 2012.
McMurtrey, K. B.; Racowski, J. M.; Sanford, M. S. "Pd-Catalyzed C H Fluorination with Nucleophilic Fluoride, " Org. Lett. 2012, 14, 4094-4097.
Kalyani, D.; McMurtrey, K. B.; Neufeldt, S. R.; Sanford, M. S. "Room-Temperature C–H Arylation: Merger of Pd-Catalyzed C–H Functionalization and Visible-Light Photocatalysis" J. Am. Chem. Soc. 2011, 133, 18566-18569.

Dr. Todd Morris
Associate Teaching Professor & Assistant Chair
Office: PSC 141, Pocatello
208-282-4373
Ph.D. Chemistry, University of Alabama, 2004
Bachelor of Science Chemistry, University of Tennessee at Martin, 1999
Recent Publications
Zangmeister, Rebecca A.; Morris, Todd A.; Tarlov, Michael J. "Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine" Langmuir, 2013, 29, 8619-8628.
Sanchez-Pomales, Germarie; Morris, Todd A.; Falabella, James B; Tarlov, Michael J; Zangmeister, Rebecca A. "A Lectin-Based Gold Nanoparticle Assay for Probing Glycosylation of Glycoproteins" Biotechnol. Bioeng. 2012, 109, 2240-2249.
Morris, Todd A. "Go Chemistry: A Card Game To Help Students Learn Chemical Formulas" J Chem. Educ. 2011, 88, 139 7- 1399.
Morris, Todd A.; Peterson, Alexander W.; Tarlov, Michael J. "Selective Binding of RNAse B Glycoforms by Polydopamine-immobilized Con A" Analytical Chemistry 2009, 81, 5413-5420.
Morris, Todd; Sun, Jia; Szulczewski, Greg "Measurement of the Chemical and Morphological Changes that Occur on Gold Surfaces following Thermal Desorption and Acid Dissolution of Adsorbed Mercury" Analytica Chimica Acta 2003, 496, 279-287.

Enouri Omar
Associate Lecturer
Office: PSC 249, Pocatello
208-282-4373

Dr. Renee Rosentreter
Senior Lecturer
Office: PSC 152A, Pocatello
208-282-4373
Postdoctoral teaching associate for the Department of Chemistry at ISU for 5 years prior to her current appointment.
Ph.D. Biopharmaceutical Analysis, Idaho State University, 1999
Master of Science Environmental Science / Waste Management, Idaho State University, 1995
Bachelor of Science Chemistry, Idaho State University, 1992
Recent Publications
Renee L. Bunde (Rosentreter), E.J. Jarvi, and J.J. Rosentreter. Review: Piezoelectric Quartz Crysral Biosensors. Talanta, vol. 46, pp. 1223-1236, 1998.
Renee L. Bunde (Rosentreter), J.J. Rosentreter, and M.J. Liszewski. The Rate of Strontium Sorption and the Effect of Variable Aqueous Concentrations of Sodium and Potassium on Strontium Distribution Coefficients. Envir0nmental Geology, vol. 34, no. 2&3, pp. 135-142, 1998.
M.J. Liszewski, Renee L. Bunde (Rosentreter), C.H. Hemming, J.J. Rosentreter, and J. Welhan. Preparation and Use of Synthesized Solutions in Batch Experiments to Determine Strontium Partition Coefficients of Surficial Sediments. Journal of Ground Water Contamination, vol. 29, pp. 93-108, 1998.
Renee L. Bunde (Rosentreter), J.J. Rosentreter, M.J. Liszewski, C.H. Hemming, and J. Welhan. Competing Ion Effects of Calcium and Magnesium on Strontium Sorption to Surficial Sediments at the Idaho National Engineering Laboratory, Idaho. Environmental Geology, vol. 32, no. 3, pp. 219-229, 1997.
C.H. Hemming, Renee L. Bunde (Rosentreter), M.J. Liszewski, J.J . Rosentreter, and J. Welhan. Effect of Experimental Technique on the Determination of Strontium Distribution Coefficients of a Surficial Sediment form the Idaho National Engineering Laboratory, Idaho. Water Research, vol. 31, no. 7, pp. 1629-1636, 1997.
Renee L. Bunde (Rosentreter) and J.J. Rosentreter. Development of a Flow Injection Analysis System Utilizing Piezaelectric Crystals for the Determination of Cyanide Employing Small Sample Sizes. Microchemical Journal, vol. 47, pp. 148-156, 1993.

Affiliate Faculty

Dr. Srinath Pashikanti
Affiliate Faculty
Office: Leonard Hall 216, Pocatello
Master of Science and Ph.D., Medicinal Chemistry, The University of Kansas
Master of Science, Chemistry & Biochemistry, South Dakota University
Dr. Pashikanti is recognized by the Chemistry Department as an Affiliate Faculty member, he is also an assistant professor in the Biomedical and Pharmaceutical Sciences Department with the College of Pharmacy.

Dr. Christopher Zarzana
Affiliate Faculty
Office: PSC 145, Pocatello
(208) 282-4373
Ph.D. Chemistry, University of Arizona
Bachelor of Science Chemistry, Harvey Mudd University
Dr. Zarzana’s research covers a broad range of topics involving mass spectrometry. He has a strong background in mass spectrometry instrumentation, especially related to ion optics and detector technology. Dr. Zarzana also has experience using chromatography-mass-spectrometry to solve a wide variety of analytical problems in complex matrices. He conducts research on ion formation and gas-phase behavior chemistry, and the effects of radiation on a variety of organic molecules.
Current research projects include: developing novel sample preparation and ionization methods and instrumentation for application to isotope-ratio mass spectrometry, development of new nanospray sources for the sensitive analysis of nano-volume samples containing radio-isotopes, and investigating the radiation chemistry of nuclear fuel reprocessing materials.

Marilu Perez
Affiliate Faculty
Office: PSC 145, Pocatello
Ph.D. Chemistry, Iowa State University
Bachelors of Science Chemistry, Idaho State University
Associates of Science Chemistry, College of Southern Idaho
