Climatology
General Climate
climates or climatic conditions.
There are several things that influence the climate of any area:
1. latitude - the farther from the equator a place is, the cooler the average temperature will be.
2. altitude (or how high an area is above sea level) - the top of a mountain is always cooler than the bottom of the mountain.
3. distance from the ocean - areas far away from an ocean have greater changes in temperature than areas close to the ocean.
4. wind - direction and speed of air currents of wind that reach an area. Idaho has extremely varied topography.
Our highest mountain, Mount Borah, rises to 12,662 feet above sea level. Our lowest valley is only 770 feet above sea level (Snake River at Lewiston). The higher altitudes have mild summer days and are extremely cold in the winter months. The lower valleys and plains seldom drop below zero degrees even in the winter. The ocean does not affect our temperature too greatly in Idaho. What it does affect is the amount of precipitation we receive. North Idaho receives large amounts of rain and snow from storms blown in from the Pacific Coast. There are no high mountain ranges to keep the clouds from that area, so precipitation may be more than four times as much as that received in the southern part of the state. Southern Idaho is protected on all sides by mountains. For that reason, much of the area receives very little rain or snow and is usually called a desert.Although t he climate varies greatly in different areas of the state, in general the climate of Idaho is temperate. Temperate means an area has four seasons with great changes in the average temperatures between summer and winter.
All areas of Idaho undergo the transition through the four seasons, but the seasons manifest differently according to geographic location. Idaho has three main geographic provinces: mountains, valleys, and plains. There is little difference in climate between the mountains and the valleys, except that the mountains shelter valleys resulting in a moderated climate compared to the mountains. Scientists call this the orographic effect; in which: wind encounters a mountain, rises and cools, loses it’s moisture, and warms while descending into the valley. The plains are semiarid flatlands that have nearly equal amounts of precipitation and evaporation. Annual highs and lows, or seasonal extremum, also vary greatly resulting in bone-chilling winters and blistering summers.
January is the coldest month of the year in Idaho; usually having average temperatures below freezing. Some areas have temperatures well below zero through much of the winter. The record low in Idaho is minus 60 degrees Fahrenheit, recorded at the Island Park Dam in 1943. Temperatures routinely drop to 20 degrees below zero in mountainous northern and eastern Idaho. Snowfall is often heavy in these areas and the annual precipitation from snow and rain is between 20 and 35 inches, respectively. However, the average annual precipitation for Idaho is 12.44 inches, a result of the very dry conditions existing on the plains.
July is the hottest month of the year in Idaho. Temperatures are highest on the plains that occupy regional lowlands: the Snake River Plain, and on the lower reaches of the Clearwater, and Snake rivers, especially from Bliss to Lewiston. The all-time record high for Idaho is 118 Fahrenheit, recorded at Orofino on July 28, 1934. Temperatures greater than 100 degrees occur each year at many locations in southwestern Idaho. For example, on July 26, 1987 the temperature at Mountain Home was 108 degrees; 104 degrees at Boise; and 102 degrees at Twin Falls.Links.
Precipitaion Patterns
There are many types of precipitation: rain, snow, sleet, and hail, to name a few. In this lesson we will learn about the mechanisms that produce various types of precipitation. It is important to note that the presence of clouds and their associated condensation nuclei alone do not always produce precipitation. Very specific conditions must occur in order for a cloud to produce precipitation.
In order for cloud droplet to form a non-equilibrium condition, where condensation exceeds evaporation, must exist. The curvature of a cloud droplet affects its rate of evaporation. The more curved the droplet, the more evaporation that occurs. Smaller cloud droplets will evaporate quickly unless the air is supersaturated (the relative humidity exceeds 100%). Because of the curvature effect, air that is saturated with respect to a flat surface is unsaturated with respect to a curved cloud droplet. An ordinary cloud droplet 100 times smaller than raindrop.
Though supersaturation is required in order for cloud droplets to sustain themselves, relative humidity rarely approaches 101%, even in very wet clouds. How do cloud droplets ever grow to raindrop size? The answer lies in the Hygroscopic nature of certain condensation nuclei. Recall that condensation on hygroscopic particles will commence when the relative humidity is below 100%. This is known as the solute effect.
Consider a parcel of air unsaturated air rich with condensation nuclei. As the air cools the relative humidity increases. At some point below 100% saturation, condensation commences on the most hygroscopic of the available nuclei. These nuclei continue to grow as the air cools further and the relative humidity approaches 100%. The curvature effect becomes negligible for larger droplets but remains appreciable for smaller nuclei. The rise in relative humidity within the air mass is slowed by the fact that the larger particles begin to remove lots of water vapor from the air. Soon, the particles are removing water vapor from the air as fast as it can be replaced from external sources. At this point the relative humidity actually begins to decrease. Condensation in clouds is such an inefficient precipitation producing process that it is very unlikely to produce, by itself, precipitation in any appreciable amount. Another mechanism is clearly responsible for producing precipitation from clouds. Two additional mechanisms are responsible for producing precipitation from clouds the collision-coalescence process, and the ice-crystal process.
The collision-coalescence process occurs in warm clouds. As cloud droplets form within clouds they become electrically charged. The cloud droplets grow larger by sticking to each other in the aftermath of collisions due to electrical attraction. As time passes the droplets grow larger and larger. Updrafts help keep the droplets suspended in the cloud longer. If the cloud is thick the droplets will also stay suspended longer. Finally, the droplets will grow large enough that they can no longer remain suspended and will begin to fall. As soon as they leave the cloud base they begin to shrink due to evaporation. Raindrops that reach the ground are smaller than those leaving the base of the cloud.
The ice-crystal process occurs in colder clouds that exist mainly in the middle to high-Iatitudes. Even in these extremely cold clouds there are liquid water droplets (existing well below freezing). These are referred to as supercooled water droplets. The temperature of a cloud, in fact, must exceed -4OoC in order for it to consist entirely of ice crystals. Such clouds are referred to glaciated.
When the temperature drops low enough within a cloud, large numbers of water molecules begin to bond in a rigid form within supercooled liquid water droplets. This leads to the formation of ice embryos, i.e., small ice crystals in the center of supercooled water droplets The water molecules must have very low rms speeds in order for ice embryos to remain intact since even slight thermal motions disrupt them. Even colder temperatures enable the crystal to become a freezing nucleus. The presence of these ice embryos enhances the freezing process. The presence of ice nuclei also enhance the freezing process. Ice nuclei may be clay (kaolinite), biological material, or anything that looks like an ice crystal. Contact freezing is another important method by which ice crystals to form in a cloud, involving collisions between ice nuclei (freezing nuclei) and supercooled droplets.
As we have seen, when precipitation first begins to fall it is usually in a frozen state. Often precipitation begins in the form of either graupel or snowflakes. Snowflakes are an aggregation of ice crystals. Much precipitation falling at middle latitudes, even in mid-summer, falls as snow flakes in the beginning. Graupel is formed by collisions between supercooled cloud droplets and ice crystals.
In a precipitation theory known as the Bergeron Process all raindrops begin as ice crystals. When the ratio of ice crystals to water droplets in clouds is on the order of 1:100,000, conditions are right for precipitation to begin. When there are too few ice crystals, the existing crystals grow large and fall out of the cloud, leaving it unaffected. When there are too many crystals, a cloud of ice crystals is formed, and no precipitation occurs because the individual crystals are all too small to fall to the ground.
Cloud seeding is an important process used quite often in the winter to create precipitation. The object is to find clouds that are deficient with ice crystals and inject artificial ice nuclei to produce the ratio of 1:100,000. (Silver iodide is usually the artificial ice nuclei used because it resembles an ice crystal so well.) A cold cloud is needed for this to work effectively.
Rain is liquid drop precipitation with diameter greater than or equal to 0.5mm.
Drizzle is a liquid drop with diameter less than 0.5mm. Virga is precipitation that doesn't reach the ground. If updrafts in a cloud change to downdrafts rainfall amount may increase to a shower. If a shower is excessively heavy it is referred to as a cloudburst.
Snow consists of frozen ice crystals falling to the ground. Because snow scatters light more effectively than rain one may easily observe where snow changes to rain below a cloud (above the freezing line is darker). If, however, one looks directly up into the precipitation from below the snow appears lighter because it scatters light in all directions below the cloud. As a result the bottom of a rain cloud appears much darker than a cloud with snow in it.
Fallstreaks are a virgalike phenomenon consisting of snow rather than rain.
Flurries are brief snow showers, typically from cumuliform clouds. A snow squall is a more intense snow shower, essentially the equivalent of a cloudburst. Continuous snowfall is associated with nimbostratus and altostratus clouds. A blizzard is a snowstorm accompanied by low temperatures, strong winds, blowing and drifting snow.
Sleet is melted snow that re-freezes into a tiny ice pellet. Freezing rain occurs when raindrops fall through a freezing layer that supercools them and subsequently freeze on contact with the ground.
Freezing drizzle is freezing rain with drop diameters less than 0.5mm. Rime is an accumulation of small, supercooled cloud droplets that are milky and granular in appearance. Snow grains and snow pellets are the solid equivalent to drizzle. Snow grains have a diameter of less than lmm and stick upon hitting a surface, while snow pellets have diameters of greater than 5mm and bounce upon hitting a surface.
Hail is produced when large, frozen raindrops, graupel, etc. act as accretion nuclei. In order for a hailstone to form, the accretion nuclei must remain in a cloud a long time and thus travel a large distance within the cloud. This process is facilitated by strong updrafts of the type common within cumulonimbus clouds. Hail is most often associated with such clouds and is therefore more common during the spring and summer than in winter. Hailstreaks are long narrow bands of land struck by hail as the precipitating cloud moves along.
About 80 per cent of the precipitation that falls on Idaho each year is in the form of snow. It takes about one foot of snow to make one inch of water when it melts. Since water is Idaho's single most important resource a system has been developed to measure snow depths in the mountains of Idaho. This system almost guarantees that water will be used efficiently, and that it will be well conserved so that everyone will have enough water each year. We all rely on the water that falls on our state each year, not just the farmers who use it for irrigation. We also use water for power, to fish in, and to help wildlife survive. Idaho's industries need water to operate and you and I need it to drink, to bathe in, to do our dishes and to water our lawns. In addition to water supply, precipitation plays a significant role in shaping the landscape around us.
The state precipitation map at left underscores the greatest natural deficiency suffered by the West. The region lacks sufficient precipitation for most of the basic needs of human beings. It has been responsible for the treeless plains and, naturally, the desert. In the Snake River Valley for example there is a yearly average of only eight inches. Where the annual amount is less than fifteen inches and irrigation is not possible, dry farming and grazing are the dominant agricultural activities
The highest amount of precipitation ever recorded in Idaho was on Deadwood Summit in Valley County in the winter of 1964-65. Precipitation of 98.6 inches was recorded that year. Much of that precipitation was in the form of snow (if it takes one foot of snow to make one inch of water when it melts, imagine how much snow fell on Deadwood Summit that winter).
Just 75 miles to the east of Deadwood Summit, Challis has the lowest average yearly precipitation in Idaho, just 7.09 inches. Of the larger cities and towns in Idaho, Boise has an average precipitation of less than 12 inches and Wallace in Shoshone County has the heaviest annual precipitation of 41.64 inches. Snow depths vary widely throughout the state, ranging from skiffs in the lower dry areas to very deep in the central mountains.
Atmospheric Moisture & Water Circulation
Atmospheric Moisture:
Water may exist in any of several phases: solid, liquid or gas, depending upon temperature and pressure. The terms evaporation and condensation are used to describe water in transition between the gaseous and liquid states. The terms freezing and melting are used to describe water in transition between the liquid and solid states.
Sublimation is a change of phase directly from the solid state to the gaseous state.
Deposition is a change of phase directly from the gaseous state to the solid state.
A saturation state is a state of equilibrium between water molecules in transition between the liquid phase and gaseous phases. The temperatures and pressures at which saturation states occur are of great importance in determining the course of most weather-related phenomena.
As we will see, water vapor, while a very small constituent of the atmosphere (percentage wise), plays a huge role in determining the course of weather and climate.
Winds enhance evaporation by transporting water vapor molecules in the air away from the site where the water is entering the air, preventing the air from becoming saturated at that site. Higher temperatures also enhance evaporation.
Water molecules in the liquid and gaseous states exist in a state of rapid motion, zipping back and forth as a result of collisions with other water molecules. In a parcel of water at a given temperature there will be a distribution of energies associated with various water molecules (i.e., not all of the water molecules will possess the same energy). The higher the temperature, the higher the mean value of the energy distribution, or the higher the average energy of each water molecule. The greater the energy of an individual water molecule, the more vigorous its motion. Water molecules at higher energies move around with greater average speeds (know as rms speeds).
Whenever water exists in the liquid state there will be at least a few molecules that have high enough energies to make the transition from the liquid to the vapor state. When the air is warm, however, many water vapor molecules will possess high enough energies to bounce around for only a short period of time before escaping the liquid state.
When the air is cooled, however, lower rms speeds allow water molecules to stick to certain objects (known as condensation nuclei) after a collision. When water sticks to condensation nuclei in the air liquid cloud droplets begin to form. Condensation is more likely when the air is cooled both because water vapor molecules can "stick" to condensation nuclei, and because lower rms speeds allow more water vapor molecules to return to the liquid state.
Circulation of Water in the Atmosphere:
The transfer of water between land, ocean and atmosphere is accomplished by a number of natural processes. Evaporation places water into the atmosphere. Transpiration, the transfer of water into the atmosphere by biological systems (primarily plants) also places water into the atmosphere. Evaporation and transpiration work together in continental areas and account for roughly 15% of the total amount of water placed into the atmosphere. The other 85% come directly from the oceans via evaporation.
Humidity
The amount of water vapor in the air is referred to as humidity. Humidity may be usefully defined in any of several ways:
* Absolute humidity is equal to the mass of water vapor divided by the total volume of air, or water vapor density. Absolute humidity changes as the volume of an air parcel changes. Changes in the volume of an air parcel occur normally as air rises or sinks.
* Specific humidity is equal to the mass of water vapor in the air divided by the total mass of the air parcel (including the water vapor). Since this is a unitless number, it is expressed as a percentage.
* Mixing ratio is the mass of the water vapor divided by the mass of the air parcel without the mass of the water vapor included (dry air). Again this is a unitless quantity that is expressed as a percentage. Specific humidity and mixing ratio remain constant as long as water vapor is not added to or removed from a parcel of air.
Specific humidity is at a maximum in the Tropics and declines as one moves poleward reaching minimum values in each polar region. This implies that the air in the most "arid" desert regions of the planet (30 degrees N or S latitude) actually contains more water vapor than temperate regions much farther north, and certainly more than the polar regions.
Vapor Pressure is the pressure exerted by water vapor molecules in the air on surrounding water molecules. In a mixture of gasses, the pressure of an individual gas is proportional to its percentage in the mixture (known as its partial pressure). The partial pressure of water vapor in a parcel of air is known as the actual vapor pressure. Normally this is a small fraction of the total air pressure. More air molecules generally means greater air pressure. Actual vapor pressure is a good measure of the total amount of water vapor in the air. As noted previously, air is saturated when there is a balance between water molecules leaving and entering the gaseous state. Saturation vapor pressure is a measure of how much water vapor is required to make the air saturated at a given temperature or the pressure that the water vapor molecules would exert if the air were saturated at a given temperature.
Relative humidity is another description of the water vapor content of air and may be stated in several different ways:
* Relative humidity = water vapor content/ water vapor capacity.
* Relative humidity = actual vapor pressure/saturation vapor pressure x 100%
* Relative humidity = actual mixing ratio/ saturation mixing ratio x 100%
Stated simply, relative humidity is the ratio of the amount of water vapor in the air to the amount required for saturation at a given temperature and pressure. A relative humidity of 100% means that an air parcel is completely saturated. It is important to note that it is entirely possible to have relative humidities > 100%. It is, in fact, necessary for relative humidity to exceed 100 % for certain meteorological processes to occur.
Although adding or removing water vapor from the air will bring about changes in relative humidity, this is not the only way that such changes can occur. A change in temperature of the air can also change the relative humidity.
Dew Point is the temperature to which a parcel of air must be cooled, at constant pressure and water vapor content, for saturation to occur. Dew point is determined with respect to a flat surface of water. The corresponding frost point is determined with respect to a flat surface of ice. Dew point is a very useful indicator of the air's actual water vapor content. High dew points correspond to high water vapor content. When air temperature and dew point are far apart relative humidity is low. When air temperature and dew point are close together relative humidity is high. Relative humidity = 100% when they are the same.
Comparing Humidities:
In the polar regions, temperatures are low, dew point temperatures are also low, and relative humidity is high. The air in polar regions is usually around 80% saturated. Even though the water vapor content of the air is low, the water vapor capacity is low as well. Saturation occurs in air that holds very little water vapor even when the air is relatively dry. As a consequence, the relative humidity is higher at polar regions than at 30o latitude, while the specific humidity is higher at 30o than at polar regions. Not all regions of the earth around 30o latitude are desert. As one traverses 30o of latitude across the continental United States, one notices a distinct difference between the climate of Arizona and that of Alabama. The air masses that prevail over the western deserts of the U.S. come from the Pacific Ocean. The Pacific Ocean is normally very cool. Air over the Pacific is also cool. As westerly winds move this air over land, its temperature increases but its water vapor content does not. Hence the air over the western deserts has a high temperature but a low dew point and very low relative humidity. The Gulf of Mexico, on the other hand, is quite a bit warmer as is the air over it. When this air moves over the southeastern U.S. it warms only a little while maintaining the same water vapor content. The air and dew point temperatures are very close and the relative humidity is consequently quite high. This is why the southwest U.S. is normally hot and dry while the southeast is hot and muggy.
Condensation
Objects near the ground cool rapidly at night through emission of infrared radiation. The ground and objects near the ground are often much cooler than the surrounding air. Air in contact with these objects cools by conduction, eventually reaching the dew point. As water vapor condenses on these surfaces deal) begins to accumulate. Dew evaporates rapidly in dry climates or windy conditions. Dew is most likely to form on calm clear nights where infrared radiation is at a maximum and movement of chilled air away from the ground is at a minimum.
Frost forms when the dew point temperature is below freezing (a.k.a. frost point). When the air temperature cools to the frost point, and further cooling occurs, water vapor can change directly to ice without becoming a liquid first in a process known as deposition.
Just as dew and frost need a surface upon which to condense, there must be airborne particles upon which water vapor can condense to produce cloud droplets. Particles known as condensation nuclei fulfill this role. Condensation nuclei provide surfaces upon which water vapor can condense to create cloud droplets. Condensation nuclei are very small (about 0.2 - 10.0 microns) particles light enough to remain suspended in the air. Condensation nuclei are formed from a variety of sources including dust, pollen, smoke, salt from ocean spray and sulfates.
There are two broad categories of condensation nuclei: hygroscopic and hydrophobic. Hygroscopic nuclei are "water seeking" nuclei. Water vapor condenses on hygroscopic surfaces readily even when the relative humidity is considerably lower than 100 percent. Salt is an example of a hygroscopic particle. Hydrophobic nuclei are water repelling. Water vapor will condense on hydrophobic surfaces only at relative humidities greater than 100 percent, and even then with great difficulty. Examples of hydrophobic nuclei are oil, gasoline, and paraffin wax. Since air usually has a mixture of both hygroscopic and hydrophobic nuclei, condensation usually occurs at relative humidities of less than 100 percent.
Haze forms when a layer of dust or salt particles is suspended in the air. In areas where haze regularly forms, visibility is enhanced when relative humidity is low because less condensation occurs on the haze particles. This keeps the particles relatively small even as the number of haze particulates remains constant. As air cools the relative humidity increases. In such an environment, when the relative humidity reaches about 75 percent, condensation begins producing a wet haze. As water collects on the available nuclei, their size increases to the point that they become effective scatterers of light, thus producing haze.
When relative humidity nears 100 percent, haze particles grow larger and condensation occurs on less active nuclei. When visibility diminishes to < 1 km, fog occurs. As fog droplets increase in size they begin to settle toward the ground. This process is fairly rapid (approx. 5 cm/sec). How then, is fog maintained? Fog forms when air is either cooled (cooled below its saturation or dew point) or by evaporation and mixing (water vapor enters the air by evaporation and the moist air mixes with relatively dry air). Once fog forms it is maintained by the continuous formation of new fog droplets on fresh condensation nuclei that replace those settling to the ground, as long as the cooling/mixing mechanism is also maintained.
Radiation or Ground Fog is a fog produced by the earth's radiational cooling. It forms best on cold, clear nights where a relatively thin layer of moist air close to the ground is overlain by drier air. The moist, shallow layer does not absorb much of the outgoing infrared radiation. The ground and the air directly above it cool rapidly, creating a surface inversion. The moist, lower layer quickly becomes saturated by this rapid cooling and fog forms. Radiation fogs are most corm-non in late fall and winter due to long nights (allows for longer cooling time) and cool air. A light breeze of less than five knots enhances the formation of radiation fog. This occurs because the slight breeze brings more moist air in contact with the cool ground, thus cooling the moist air layer more efficiently. A strong breeze squelches this process by mixing the moist surface air with the drier air above. You may have noticed that fog tends to dissipate or "burn off" after the sun has been up for a short while. This occurs because some sunlight penetrates the fog and begins to warm the ground thus disrupting the fog creating cycle.
Advection fogs form as the result of wind moving moist air from above a warmer surface to a region above a cooler surface. The moist air cools to its dew point after coming in contact with the cooler surface producing fog. Advection fogs are typical in most coastal regions. During summer months, moist air is carried by the wind from warmer waters (near the surface) offshore. When this warm air reaches cooler surface waters near the coasts the air cools and condenses creating a fog. Advection fogs often provide an important moisture source for surrounding biological systems in coastal regions.
Upslope fogs form when moist air flows upslope in mountainous regions. With this upward movement the air cools to it's dew point causing condensation and fog formation. Upslope fog is common on the eastern side of the Rocky Mountains. Upslope fogs can persist for many days in some regions.
Evaporation or mixing fog is the mixing of two unsaturated air masses to produce a fog. If moist air meets and mixes with cooler air resulting in saturation, a fog forms. Mixing fogs are common in ski areas during times of rainfall. As rain falls onto the snow it begins to melt. The melting process extracts heat from the surrounding environment, including the air close to the ground. For readily forms in the cool, rain-saturated air. When you breathe condenses in front of your face on a cold morning you are staring at another example of a mixing fog.
In the U.S., heavy fog is more prevalent in coastal regions than in the center of the continent. Three major regions stand out as having the most days with heavy fog:
1. Pacific Coast region
2. Appalachian Highlands
3. New England region
Fog often causes poor visibility. Driving from a clear area into fog on a major freeway can be extremely dangerous. This problem is exacerbated when driving at night with high-beam lights on. This is because the light from lamps is reflected backward by the fog droplets. It is much better to use low beams in this situation to change the angle of the reflected light away from the level of the driver's eyes. People who frequently drive in foggy areas often install fog lamps. Fog lamps direct light downward toward the road surface. In addition to solving the reflection problem, fog lamps illuminate a drier layer of air that typically exists within a foot or so of the road providing better visibility.
Clouds
There are ten basic types of clouds categorized by both their appearance and their height above the earth's surface. The four major cloud groups are high, middle and low clouds, and clouds with extensive vertical development.
|
Examples of different cloud types
|
||
|
Altostratus
|
Altocumulus
|
Cirrostratus
|
|
Cirrocumulus
|
Cirrus
|
Cumulonimbus
|
|
Cumulus
|
Nimbostratus
|
Nimbus
|
|
Stratocumulus
|
Stratus
|
|
High clouds generally form above 6000 m. Because the air is cold and dry, high clouds are composed almost exclusively of ice crystals. These clouds also tend to be very thin. Cirrus (Ci) clouds are the most common of this group and can be blown into long, wispy streamers often called mare's tails. Cirrus clouds have little vertical development. Cirrocumulus (Cc) clouds are an infrequently seen type of high cloud. These clouds can occur individually or in long rows and often have a "rippled" appearance. This rippling looks like fish scales in the sky and the appearance of Cc clouds is sometimes referred to as a mackerel sky. These clouds are composed mainly of ice crystals and have little vertical development. Cirrostratus (Cs) clouds are common high clouds that have a sheetlike appearance. Cs clouds have varying vertical development and are cold and dry. Thick cirrostratus clouds indicate impending bad weather. Halos are usually associated with cirriform clouds.
Middle clouds have bases between 2000 and 7000 m. These clouds are composed of water droplets and ice crystals (if temperature is low enough). Altocumulus (Ac) clouds are puffy and can be found in varying shades of gray. They are mostly water droplets and have moderate vertical development. Altostratus (As) clouds are a combination of water droplets and ice crystals that are gray to blue gray in color. Altostratus clouds usually block out a majority of sunlight light and result in gray, dreary days.
Low clouds, with their bases lying below 2000 m, are almost always composed of water droplets. The Nimbostratus (Ns) clouds are dark gray and are associated with continuously falling rain or snow. Nimbostratus clouds can occupy the entire sky and have moderate vertical development. Ns clouds are associated with stratus, fractus, and scud clouds. Stratocumulus (Sc) clouds are low, rounded, and "lumpy" with small patches of blue sky in between. These clouds are much larger elements than altocumulus but are not associated with precipitation. Stratus (St) clouds are typically a uniform grayish color covering over the entire sky. These clouds are often mistaken for a fog, but they do not touch the ground. They may be associated with small amounts of precipitation, such as drizzle, but do not produce larger precipitation.
Clouds with large vertical development include the puffy, "floating cotton" appearance of the cumulus (Cu) cloud is a common sight. These clouds can be distinguished from a stratocumulus cloud by the large amounts of sky visible between each cloud, compared to the relatively small space between the Sc clouds. There are three sub-groups of the cumulus cloud group:
1. Cumulus humulis - has little vertical development
2. Cumulus congestus - is a towering cumulus
3. Cumulonimbus (Cb) - has a great deal of vertical development, also known as thunderstorm clouds
Other miscellaneous cloud types include:
Lenticular clouds - mountain waves, banner clouds
Pileus clouds - these clouds are formed when moist winds are deflected up and over cumulus clouds
Mammatus clouds - produced by moist, sinking air cooler than the surrounding air mass
Contrails - sometimes produced by aircraft
Nacreous clouds - found in the stratosphere
Noctilucent clouds- clouds found in the upper mesosphere.
Stability & Cloud Development
Cloud development is linked closely with the concept of stability, i.e., the tendency of air to rise. Although several factors determine whether or not clouds will form, the stability of the atmosphere is far and away the single greatest indicator of cloud formation.
Air tends to cool and condense as it rises, and to become warm and dry as it sinks. A Parcel of Air is an imaginary mass of air that doesn't exchange properties with surrounding air masses. In reality air masses do exchange properties, but this often occurs very slowly, especially if the air masses are large. An adiabatic process is one where no heat is exchanged between an air parcel and the surrounding air. When we talk about an adiabatic process in the current context we are talking about a rising (or sinking) parcel of air that is not exchanging any heat with its surroundings. When air rises it cools at a relatively constant rate. If the air is unsaturated, this rate, called the dry adiabatic rate, is 10°C per 1000m (5.5°F per 1000ft), i.e., a parcel of unsaturated air cools by 100C every 1000 meters if it doesn't exchange heat with its surroundings.
As air rises and cools its relative humidity increases. At some point the dew point equals the air temperature and the air becomes saturated. Further lifting results in condensation and cloud formation with an accompanying release of latent heat into the rising parcel of air (remember that condensation is a warming process). Because the heat liberated by condensation partially offsets the cooling due to expansion, the parcel now cools at a lesser rate as it rises. This rate is known as the moist adiabatic rate. The moist adiabatic rate applies to saturated air.
On average, the moist adiabatic rate is less than the dry adiabatic rate. The moist adiabatic rate is not constant but varies with temperature and moisture content. For cool air the moist adiabatic rate ~ dry adiabatic rate. For warmer air the moist adiabatic rate is less than the dry adiabatic rate. An average value of 6°C per 1000m (3.3°F per 1000ft) is commonly used.
To determine the stability of an air parcel, one compares its temperature to the temperature of the surrounding air mass. If the air parcel's temperature is less than the temperature of the surrounding air mass, it is denser than the surrounding air and therefore has a tendency to sink. Air that has a tendency to sink is known as a stable air. If the air parcel's temperature is greater than the temperature of the surrounding air mass, the air parcel is less dense and tends to rise. Rising air, as we have already learned, is known as unstable.
For stable air, the environmental lapse rate is 4°C per 1000m (2°F per 1000ft). When the environmental lapse rate is less than the moist adiabatic rate an air parcel cools more quickly than the surrounding air mass. This is known as absolute stability. ln this case the air parcel strongly resists lifting. If the parcel is forced to lift by mechanical means (such as orographic uplift or uplift along a frontal boundary), it will spread out horizontally. Any clouds that form as a result will be thin and horizontal such as cirrostratus, altostratus, nimbostratus, and stratus clouds. All of these cloud types are associated with stable air.
Since the moist adiabatic rate must be less than the environmental lapse rate for stable conditions to exist, a moderate to small environmental lapse rate enhances stability in the atmosphere. Warm air aloft (caused by warm advection) and cool air at the surface (caused by nighttime radiational cooling, cold advection, or a cold surface) result in a moderate to small environmental lapse rate.
Fog and haze form in stable atmospheric conditions because of the large scale sinking of air. This can form an inversion condition, known as a subsidence inversion.Subsidence inversions are often associated with large high-pressure systems. Inversions are absolutely stable because the air beneath the inversion is physically impeded from moving upward. This traps large numbers of particulates close to the ground that serve as fog-forming condensation nuclei.
Neutral Stability is an atmospheric condition that occurs when the environmental lapse rate is equal to the dry adiabatic rate.
Absolute instability occurs when dry adiabatic rate is less than the environmental lapse rate. In this situation, an air parcel will be warmer and less dense than the surrounding air and will rise due to buoyant forces. Clouds with extensive vertical development are indicative of absolute instability.
Conditional instability is a state of instability that depends upon whether or not the rising air is saturated.
Conditional stability occurs when the environmental lapse rate is between the moist and dry adiabatic rates. The atmosphere is normally in a conditionally unstable state.
Many factors lead to instability. One is a steep environmental lapse rate resulting from cool air aloft (brought on by cold advection, the environmental lapse rate or both) coupled with warm air at the surface (caused by daytime solar heating, warm advection, or a warm surface).
Mixing is another factor that affects instability. Mixing increases warming below and cooling higher up in the atmosphere.
Another factor that enhances instability is lifting. When a layer of air is forced to rise it tends to become more unstable because the top layer cools more rapidly than the bottom. This steepens the environmental lapse rate. This effect is enhanced even more when the lower layer of the lifted parcel is moist and the upper layer is dry. In this case, less lifting is require to steepen the environmental lapse rate. This is referred to as convective instability and is associated with severe storms.
Cloud Development:
The lifting mechanisms associated with cloud development are:
* Surface heating and free convection
* Topography
* Convergence of surface air
* Uplift along fronts
* Convection (thermal development)
As the surface of the earth heats up due to incoming solar radiation, warm bubbles of air (thermals) develop and begin to rise. When these thermals reach a height known as the condensation level, cumulus clouds begin to form. Cooler air surrounding the clouds sinks to replace the warm air rising from the surface. The subsidence outside of the cumulus clouds suppresses cloud formation in the area surrounding the clouds. This is why one normally sees lots of blue sky surrounding fair-weather cumulus clouds. Eventually, a growing cumulus cloud cuts off the ground from the sun's rays, reducing surface heating and convection. The cloud begins to dissipate and the process may start again. Fair weather cumulus clouds are often characterized by level bases (at the condensation level), moderate vertical development, and lots of blue sky in between.
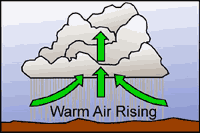 Convective cloud formation is an adiabatic process, i.e., there is little mixing between the air parcel and the surrounding air mass. A single thermal is all that is necessary to produce a cumulus cloud. Cloud formation occurs when the relative humidity within the parcel of rising air reaches 100%. After this point, the parcel remains saturated as it rises.
Convective cloud formation is an adiabatic process, i.e., there is little mixing between the air parcel and the surrounding air mass. A single thermal is all that is necessary to produce a cumulus cloud. Cloud formation occurs when the relative humidity within the parcel of rising air reaches 100%. After this point, the parcel remains saturated as it rises.
Suppose that the temperature of a rising air parcel is 350C and the dew point 270C. At first the parcel is buoyant and rises freely. The rising air in the parcel expands and cools at the dry adiabatic rate. The dew point also drops but not as rapidly as the air temperature due to the decrease in air pressure. As the unsaturated rising air cools, the air temperature and dew point approach each other increasing the relative humidity. At some height saturation occurs followed by condensation and the formation of clouds. Above this level the air cools at the moist adiabatic rate, condensation continues, and the dew point drops with height even more rapidly than before. The temperature and dew point decrease at the moist adiabatic rate.
The rising air parcel remains warmer than the surrounding air mass and continues to rise. Eventually the air parcel has a temperature equal to the air mass temperature and spontaneous lifting ceases. Stability above the condensation level plays a major role in determining the height of cumulus clouds. The stratosphere is quite stable. Seldom do clouds penetrate the tropopause into the stratosphere. Vertical development of a cumulus cloud also depends upon entrainment. If the environment around the cloud is dry (the usual case) cloud droplets will evaporate when exposed to the drier air. Entrainment occurs when this resulting cool air mixes with the convective cloud, increasing the rate at which the rising air cools. In this case the cloud may cease vertical development even though the lapse rate indicates conditional instability.
 Orographic uplift occurs when air is forced upward over a topographic barrier. If the barrier is tall enough, the air parcel is forced upward to the lifting condensation level where clouds are formed and precipitation may occur. Depending on conditions, air parcels can be forced back down on the lee side of mountains (warming and drying as they sink and compress) or can continue to rise, forming cumulus clouds. In mountainous regions this often results in a rain shadow. When clouds form over western Oregon and Washington, for instance, they produce copious amounts of precipitation as the prevailing winds forced them up and over the western side of the Cascade Range, leaving them dry on the eastern side of the mountains. The dry areas of central Oregon and Washington are in the rain shadow of the Cascade Range.
Orographic uplift occurs when air is forced upward over a topographic barrier. If the barrier is tall enough, the air parcel is forced upward to the lifting condensation level where clouds are formed and precipitation may occur. Depending on conditions, air parcels can be forced back down on the lee side of mountains (warming and drying as they sink and compress) or can continue to rise, forming cumulus clouds. In mountainous regions this often results in a rain shadow. When clouds form over western Oregon and Washington, for instance, they produce copious amounts of precipitation as the prevailing winds forced them up and over the western side of the Cascade Range, leaving them dry on the eastern side of the mountains. The dry areas of central Oregon and Washington are in the rain shadow of the Cascade Range.
Lenticular clouds and standing wave clouds are formed by topography. These clouds are associated with mountain ranges. Rotor clouds can form in standing wave clouds. These are very dangerous in the world of aviation due to the presence of wind shear.
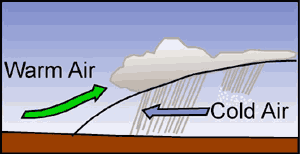 Just as mountain ranges influence cloud formation by mechanical lifting, widespread convergence of air masses also forces air upward causing cloud formation. Low-pressure systems are associated with converging air. The presence of a low-pressure system is normally associated with extensive clouds and precipitation.
Just as mountain ranges influence cloud formation by mechanical lifting, widespread convergence of air masses also forces air upward causing cloud formation. Low-pressure systems are associated with converging air. The presence of a low-pressure system is normally associated with extensive clouds and precipitation.
The gradual Iifting of stable air as a warm front passes can cause formation of stratus clouds over hundreds, even thousands of square miles. Cold fronts are much steeper and as a result, air is forced to rise more abruptly. Cold fronts are associated with instability and cumuliform clouds.
Clouds often change form. A temperature change within a cloud may cause this. Clouds are good absorbers of infrared radiation. Usually the tops cool rapidly due to increased radiation and bottoms warm due to increased absorption. This leads to instability, convection, and in turn, to a change in cloud form.
Clouds Over Idaho
All types of storms occur in Idaho including tornadoes, violent electrical storms, torrential downpours, blizzards and more. Local and regional weather in Idaho is highly changeable. A common rule-of-thumb is that if you don't like the current weather, look again in 5 minutes. The state's rugged topographic relief, geographic position relative to the jet stream, and its location within the rainshadow of the Cascades and northern Sierras, all contribute to this variability Every kind of cloud formation is visible within the state as you travel from the plains to the high mountains.
Of particular interest are the giant cumulonimbus systems which build over the Snake River Plain. The flat, open surface of the Plain heats readily during daylight hours, and convective influence on local weather is very high. Thunderheads form readily - from singular clouds to vast storm fronts stretching across the Plain.
These storms typically migrate from the southwest to the northeast, and produce spectacular lightning storms. The storms are also influenced by the hills and mountains bordering the edge of the plain.
Atmospheric Structure
The atmosphere has 4 distinct layers: 1) Troposphere, 2) Stratosphere, 3) Mesosphere, and 4) the Thermosphere. These layers are distinguished from one another by the a) mass budget [total amounts and types of elemental and molecular species], and b) changes in air motion that occur within each layer c) changes in temperature with change in altitude. These changes in the physical characteristics of air exhibit how Earth interacts with the Sun to produce our atmosphere, control our climate, distribute elements and molecules, and protect the biosphere of Earth.
The troposphere contains approximately 75% of the total mass of the atmosphere including essentially 100% of the water vapor and particulate matter. The average thickness of the troposphere is 7 miles, ranging from 5 miles thick at the poles to 11 miles at the equator. The air constantly overturns in the troposphere in regular patterns as a response to the amount of energy received from the sun; thus distributing heat, moisture, and particulate matter (pollution, volcanic debris) about Earth’s’ surface. Temperature decreases uniformly with positive changes in altitude at a rate of ~ 16° C per mile. Therefore the temperature at the top of the troposphere is, on average, 112° C colder than the surface.
Above the troposphere is a layer of constant temperature, the lower stratosphere. The stratosphere has two temperature regimes, in which the upper stratosphere differs by showing a slight temperature increase with positive changes in altitude. This temperature inversion is the result of two factors: 1) the air has little input of cool moisture from the troposphere, and 2) a combination of warming from the sun with the low air overturn rates create an insulating affect where less energy penetrates to the lower stratosphere. Solar energy also creates ozone in the stratosphere. Ozone production is concentrated near the lower and upper stratosphere boundary at about 15 miles above Earth’s surface. The upper boundary the stratosphere is nearly 28 miles above Earth’s surface, and therefore is a 15-20 mile thick lid of heated air that acts as a barrier to space and the troposphere. The stratosphere is a significant barrier because its static air masses help contain the troposphere’s moisture and buffers solar energy.
Above the stratosphere is the mesosphere. The mesosphere is rather unremarkable except that air temperatures reach a chilling -93° Celsius at the top. It is a 28-mile thick column of air that extends to a distance of 55 miles above Earth. The physical characteristics of air change dramatically above mesosphere that result from the extremely low atmospheric pressures acting on the mixture of gases we call air.
The thermosphere extends from the mesosphere to the outer edge of the atmosphere. Here low pressures result in expanded gases that do not interact, otherwise known as thin air. The thermosphere is so called because it was predicted that temperatures would be very high here do to solar inputs, but because there is few molecules available to trap energy it is like heating a large room with a handful of sparks spread about that room. Two popular physical phenomena occur in the thermosphere: Aurora Borealis, and aurora Austrialis. These phenomenon occur at the north and south poles, respectively; and are a result of the input of solar particles cascading through the thermosphere. Solar particles are charged ejecta from the sun, which can be concentrated in showers during solar magnetic storms. In northern latitudes we call this glowing solar rain the northern lights.
The four inner spheres of the atmosphere connect at zones of transition that are about a mile thick. A transition zone is called a pause, because a pause in normal behavior occurs there. Therefore, the tropopause marks the border between the tropopause and the stratosphere and so on for the stratopause and the mesopause. The outer border of the thermosphere is marked by a fringe contact of our atmosphere and space, the ionosphere. Unfiltered x-rays, that is not interfered with by air or mass, and ultraviolet energy from the sun ionize the atoms of gases in this fringe zone. The resultant charged atoms bounce back all radio signals having a shorter wavelength than 30MHz. Thus short wave communications may occur over long distances, and the rest like television signals pass into space.
Atmospheric Chemistry
The atmosphere can be described in terms of three physical components, dry gases, water vapor, and solid particles. The atmosphere is chemically stratified and 99% of the mass in the atmosphere in contained with in the troposphere and stratosphere alone. This means that 99% of the mass is contained within the inner 10% of the atmosphere. The skewed distribution is due to waning gravitational effects with distance from earth, combined with linearly decreasing atmospheric pressures acting on the gases. Therefore, most discussion about mass budgets, and the chemical make up of the atmosphere focus upon the inner two spheres.
Air itself may be described as a mixture of dry gases, water vapor, plus or minus solid particles and it’s composition changes with distance from earth. Nitrogen, oxygen, argon, and carbon dioxide are 99.9% of the natural dry gas composition of air. Beyond the Stratosphere, the gases are distributed according to their densities creating stratified molecular layers with the heavier molecules nearer to Earth. The stratification remains undisturbed in general because it resides in the static upper levels of the atmosphere, rarely mixing beyond the pausal zones. Solar radiation interacts with the chemical strata, producing charged particles by stripping electrons. Ozone is a byproduct of electron stripping, which allows O2 to become O3. Fortunately, this occurs only high in the atmosphere, because ozone in high concentrations is quite hazardous.
Oxygen is a colorless, odorless gas first discovered by Joseph Priestly, an English Clergymen. Oxygen is essential to all air breathing animals, respiring plants, and accounts for 89% by weight of all the water and air within Earth’s planet system. Oxygen is highly reactive due to its electron valence. Because oxygen is highly abundant and very reactive many oxides form on Earth and in its’ atmosphere. That’s why airborne pollutants cause such problems, we emit excess carbon, nitrogen, and sulfur, into the air which oxidizes and remains suspended in the air poisoning the biosphere.
Carbon dioxide is a colorless, odorless gas that is noxious to animals, but necessary to plants. Plants use carbon dioxide, water, and energy derived from the sun to produce sugar. This process is photosynthesis. Carbon dioxide may be considered a major pollutant though, when human activities put enough carbon into the atmosphere to cause immediate health hazards, local climate changes, and possible long term climatic effects. Carbon monoxide is a byproduct of two major consumer products: 1) cement manufacture, and 2) fossil fuel consumption. Because carbon monoxide can reflect short wave radiation emitted from earth towards space, it can cause increases in local temperatures. Scientists also theorize that if catastrophic concentrations of carbon dioxide accumulate in the atmosphere our global temperatures will rise causing catastrophic unrecoverable global climate changes. Opposing scientists argue that the carbonate cycle would buffer Earth’s atmosphere by precipitating limestone in the worlds oceans, seas, and lakes.
What is the Jet Stream?

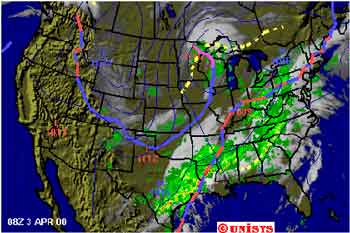
The jet stream is a stream of wind that flows high above the earth, and moves from east to west over our state. Storms out over the ocean change how the jet stream passes over us and where it comes from. Sometimes the jet stream flows southward from Alaska and Canada. When it does, it brings colder weather to Idaho. At other times, storms off the coast of California and sometimes as far away as Hawaii send warm, moist air over our state and cause our weather to change. The next time you watch a weatherman on television, listen to how he explains why our weather will be what it is. Usually, he will talk about how the jet stream affects our weather. In this image supplied by AccuWeather, we can see the jet stream as a blue line.
What is the Electromagnetic Spectrum?
Electromagnetic radiation is a form of energy emitted by all objects. Light rays are a form of electromagnetic radiation. Other rays in the electromagnetic spectrum are invisible to humans, such as television and radio waves, microwaves, X-rays, and gamma rays.
The electromagnetic spectrum is made up of waves of different lengths, forming bands. All of these waves have their own distinct set of measurements or properties: Speed, amplitude, wavelength and frequency are used to measure a wave. All electromagnetic waves travel at the same speed, 186,000 miles (300,000 km) per second, through a vacuum. This includes visible light.
A wave’s amplitude is the distance from the middle of the wave (or its resting position) to the crest or trough. The amplitude squared is proportional to the amount of energy the wave carries. In other words, a wave with twice the amplitude of another carries four times the energy.
The wavelength of a wave is measured from the crest of one wave to the crest of the next one, or from the trough of one to the trough of the next. The crest is the highest point of the wave and the trough is the lowest point of the wave. Radio waves are long waves, and can measure thousands of yards long from crest to crest. Microwaves have wavelengths of only a few millimeters, and cosmic rays are only a tiny fraction of a millimeter in length.
The frequency of a wave is its rate of vibration, or put another way the frequency of a wave is the number of crests (troughs) that pass a given point in one second. One wave per second equals one hertz. If four water waves pass the edge of a pier in 20 seconds, the frequency is four waves divided by 20 seconds, or 0.2 hertz. Waves with short wavelengths have high frequencies, while waves with long wavelengths have low frequencies.
Visible light, that which we see, forms the middle band of the spectrum. Light is unusual in that it behaves as a wave and a particle. The particles of light are made of energy, called photons, and they are able to knock electrons out of place in an atom just as a billiard ball knocks another out of place in a game of pool. Light also travels in a straight line as a wave made of a magnetic field and an electric field, which are oriented at right angles to each other.
Most objects look the color they are because of the way their structure reflects light. An object that reflects light of all wavelengths in equal amounts looks white. An object that absorbs shorter wavelengths of light but reflects longer waves looks red. An object that absorbs all wavelengths in equal amounts appears black.
Weather vs. Climate
What's the difference?
Our weather changes from day to day. Some days it may be rainy, the next day might be sunny. Sometimes we have a few days that are cold, then the temperature might warm up for a while. Weather is the daily or weekly changes in wind, moisture and temperature of an area. All three of these changes take place in the air around us. We call the air around the earth the atmosphere.
Weather in an area over a period of years is called climate. In some climates the winters are long and cold. In other climates the winters are short and mild.
It is important to understand climate. Climate affects the clothes we wear, how we heat our houses and the plants we grow. Water vapor is part of climate because it is the moisture in the air. Have you wondered where water goes when it dries from the sidewalk after a rain? It evaporates or changes into a gas. It goes into the air and stays invisible until it condenses, or changes from gas to liquid. It usually condenses to form a cloud.
What is Radiation?
Radiation is everywhere. It is a form of energy. Radiation is produced when the nucleus of an atom is broken apart. Huge amounts of energy are stored in the nucleus. When it splits (called fission) it produces heat and radiation. Nuclear power plants use the heat to turn water into steam and produce electricity. The radiation, and any radioactive materials generated are considered to be waste products. More than four-fifths of the radiation we receive comes from natural sources. This is called background radiation because it is present everywhere, all the time. Radioactive atoms are often called radionuclides.
Radioactive Decay & Half-Life
When atoms split apart naturally it is called decay. Decay happens because certain elements have unstable nuclei - a situation occurring because they have a whole lot of neutrons and so the nuclear forces are not balanced. 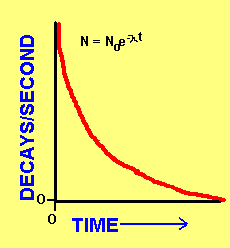 When an atom decays it can form a different isotope of the same element (i.e. it loses neutrons) or it can become a new element (i.e. it loses protons). It is important to realize that, just because atoms have experienced one decay, it does not mean that they are no longer radioactive. Some elements decay multiple times and go through a series of elements that are all radioactive. Eventually though, enough time will pass and a stable elemental form will be reached. An example of this is the decay series of which Radon Gas is an intermediate. This series has multiple decay steps from the original Uranium atoms to final, stable lead isotopes.
When an atom decays it can form a different isotope of the same element (i.e. it loses neutrons) or it can become a new element (i.e. it loses protons). It is important to realize that, just because atoms have experienced one decay, it does not mean that they are no longer radioactive. Some elements decay multiple times and go through a series of elements that are all radioactive. Eventually though, enough time will pass and a stable elemental form will be reached. An example of this is the decay series of which Radon Gas is an intermediate. This series has multiple decay steps from the original Uranium atoms to final, stable lead isotopes.
One important property of radioactive elements is half-life. Half-life is defined as the amount of time it takes for one half of all of the atoms of that element to experience a decay. Because you are halving the number of atoms each time, the amount of atoms of a particular element (and so the total number of decays) decrease exponentially! N is the number of decays/second (or atoms) at a particular time t. No is the initial amount. Lambda is a decay constant.
As an example, lets say you start with 1000 atoms of a radioactive element that has a half-life of 10 years. In 10 years you will have 500 atoms; in 20 years you will have 250 atoms; in 30 years you will have 125 atoms and so on...until they have all decayed.
Half-life is an extremely important property of radiation. It is used to determine how long a particular radioactive species will remain radioactive. That is, it tells how long that species may present a danger to human health and the environment. Half-life varies widely from element to element. For example Uranium-235 has a half-life of 704 million years while Oxygen-9 has a half-life of only 26.9 seconds! Half-life is an important consideration when attempting to determine how to dispose of a particular radioactive waste. This is because the waste must be contained for as much time as it takes to render it harmless. In general, a radioactive species is considered to be harmless when it reaches background levels - usually after about 10 half-lives.
Sources of Radiation
Radiation is a natural part of our every day lives. There are all kinds of sources of radiation. Some of these are:
- RADON GAS -this is a significant natural source.
- COSMIC RAYS -can act on nuclei in the atmosphere to produce radionuclides.
- COMBUSTION OF FOSSIL FUELS -fly ash, produced during the combustion of coal, contains a significant amount of radionuclides, even more than an equivalent nuclear power plant!
- NUCLEAR REACTORS / REPROCESSING SPENT FUEL -this is a significant source of Krypton-85 in the atmosphere.
- NUCLEAR WEAPONS -the above ground detonation of nuclear weapons releases tremendous amounts of radionuclides into the atmosphere.
In addition, people are exposed to radiation from manufactured sources such as color televisions and smoke detectors.
Types of Radiation
Radiation can occur either as high speed particles or as waves. Non-ionizing radiation is low energy radiation and cannot alter atoms. Examples of non-ionizing radiation are microwaves, infrared, and visible light. Ionizing radiation is high energy radiation that is capable of altering atoms.
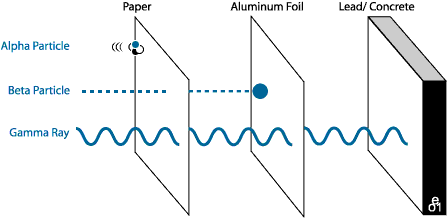
ALPHA PARTICLES - the slowest, least energetic type. They do not travel far, and can be blocked by almost anything. In general alpha particles cause damage only if inhaled or swallowed.
BETA PARTICLES - are more energetic. Beta particles can pass through cloth or paper, but can be stopped by a sheet of foil, or glass. Like alpha particles, beta particles generally are harmful only if inhaled or swallowed. For example, Radon gas emits beta particles, and is harmful because it is inhaled.
GAMMA RAYS - are the most energetic, and dangerous. These are rays, not particles and they can pass through almost anything. It takes very, very thick concrete, lead or steel to block gamma rays. This type of radiation is dangerous and damaging.
Each radioactive element emits its own characteristic form of radiation.
Types of Radioactive Waste:
LOW LEVEL WASTES - do not present a significant hazard. They emit low levels of radiation, usually in the form of alpha particles, and generally have short half-lives. These wastes tend to be suitable for burial given a 500 year containment. Some examples of low level waste are solution residuals from processing operations, contaminated equipment, contaminated clothing etc. The historically preferred method for disposing of low level waste s was dilute and disperse. This is no longer an acceptable practice.
HIGH LEVEL WASTES - These wastes are very radioactive and present a real threat to human health and the environment. They tend to be beta or gamma (or both) emitters. High level wastes are problematic to store, contain and dispose of. Examples of high level wastes are the fission byproducts in spent fuel rods and fuel assemblies from nuclear reactors such as Krypton-85 (gamma and beta emitter;half-life=10.72 years), Strontium-90 (beta emitter;half-life=29.1 years) and Cesium-137 (gamma emitter;half-life=30.17 years).
TRANSURANIC WASTE - Transuranic wastes can be low level, high level or both, and consist of radioactive materials with atomic numbers greater than 92 (they have greater than 92 protons).
Another type of radioactive waste is MIXED WASTE which is a combination of radioactive and hazardous waste (for regulatory purposes these are taken as two different things
SOURCE - U, Th, or any other material that is determined by the AEC to be a significant source of radioactivity. In particular, they regulate ores containing U, Th or other radioactive species in concentrations high enough to be useful for the manufacture of nuclear power or nuclear weapons
SPECIAL - any radioactive material that the AEC designates as significant enough to regulate, but that is not source material.
BYPRODUCT - any other naturally radioactive material , or material made radioactive via exposure to a radiation incident produced during such processes as the manufacturing of weapons, reactors, mining source material etc.
Climate Station Tour
 Weather stations have been operating in the United States since at least 1849 when The Smithsonian Institution began supplying weather instruments to telegraph companies. Weather data collection later became the responsibility of the U.S. Army and is now under the control of the National Weather Service, a division of the Department of Commerce.
Weather stations have been operating in the United States since at least 1849 when The Smithsonian Institution began supplying weather instruments to telegraph companies. Weather data collection later became the responsibility of the U.S. Army and is now under the control of the National Weather Service, a division of the Department of Commerce.
Early weather data collection was done manually by people on the ground. Forecasters, who needed more information about what was happening in the skies, began using kites to measure temperatures above the ground. They would simply tie thermometers or other instruments to the kite. After letting it fly for a while, they would reel in the kite and record data taken by the instruments.
Temperature Dewpoint Sensor
 The Temperature/Dewpoint Sensor (hygrothermometer) measures the temperature and dewpoint of the ambient air.
The Temperature/Dewpoint Sensor (hygrothermometer) measures the temperature and dewpoint of the ambient air.
Dewpoint is the temperature to which a given parcel of air must be cooled at constant pressure and constant water-vapor content in order for saturation to occur (and condensation to occur).
Hygrothermometer is an instrument that is used to obtain ambient temperature and dewpoint from remote sensors. The readouts are usually located inside the weather office
The sensor uses a chilled mirror method to measure dewpoint. In this method, a mirror is cooled to the point where a fine film of condensate is present on the mirror's surface. The temperature of the mirror at this condition is equal to the dewpoint temperature. The presence of condensation is detected by the reflection of an infrared light off the surface of the mirror. Internal circuits refrigerate a small mirror, and by using an optical feedback loop, maintain the mirror at exactly the temperature at which the mirror surface is slightly clouded with condensed water vapor from the sampled air. A thermal sensor embedded in the mirror measures the temperature. A similar thermal sensor in a sample of the ambient air measures the ambient air temperature.
The measured temperatures are transmitted to the Data Collection Package (DCP) using a power cable and two fiber optic cables.
Rain Gauge
(LIQUID PRECIPITATION ACCUMULATION SENSOR)
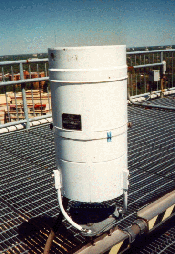 The Liquid Precipitation Accumulation Sensor (Rain Gauge) measures the amount of liquid precipitation. The precipitation accumulation sensor is a heated tipping bucket rain gauge.
The Liquid Precipitation Accumulation Sensor (Rain Gauge) measures the amount of liquid precipitation. The precipitation accumulation sensor is a heated tipping bucket rain gauge.
The Rain Gauge is a free-standing receptacle for measuring precipitation. It contains an open top, which measures approximately one foot in diameter. The sensor allows precipitation to fall into the upper portion, which is called the collector. The collector is heated to melt any frozen precipitation, such as snow or hail, for collection. Collected water is funneled to a mechanical device (tipping bucket), which incrementally measures the accumulation and causes the momentary closure of a switch for each increment. The tipping bucket is designed to measure in increments of 0.01 inch of rain. As water is collected, the tipping bucket fills to the point where it tips over. This action empties the bucket in preparation for additional measurements, closes the momentary switch, and signals another 0.01 inch of precipitation to the Data Collection Package (DCP). Water discharged by the tipping bucket passes out of the rain gauge through a lower funnel to the ground below.
At specified sites, a 48-inch diameter wind shield is installed around the rain gauge. The wind shield reduces wind updrafts and wind streamlines that alter rain trajectories.
Cloud Height Sensor
(CEILOMETER)
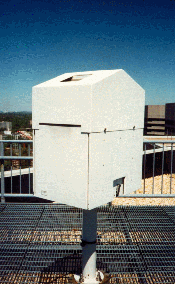 The Cloud Height Sensor (the ceilometer), determines cloud height and levels in the atmosphere. The ceilometer (pronounced se-lom'-i-ter) uses invisible laser radiation to detect cloud levels. The ceilometer works by transmitting a pulse of laser light into the atmosphere and sensing the light return as it is reflected back toward the ceilometer by objects in its path. By timing the interval between the transmission and reception, the height of particles (such as water droplets or ice crystals in clouds) above the ceilometer is calculated and reported to the Data Collection Package (DCP).
The Cloud Height Sensor (the ceilometer), determines cloud height and levels in the atmosphere. The ceilometer (pronounced se-lom'-i-ter) uses invisible laser radiation to detect cloud levels. The ceilometer works by transmitting a pulse of laser light into the atmosphere and sensing the light return as it is reflected back toward the ceilometer by objects in its path. By timing the interval between the transmission and reception, the height of particles (such as water droplets or ice crystals in clouds) above the ceilometer is calculated and reported to the Data Collection Package (DCP).
The ceilometer measures how much time it takes a pulse of light to travel from the Ceilometer Transmitter, through the atmosphere, to reflect off a cloud, and to return to the Ceilometer Receiver. The distance traveled by a light pulse can be calculated, by starting a timer when the transmitter sends out a pulse of light and by stopping the timer when the receiver detects the return of the reflected pulse. Because the total distance traveled includes a path from the transmitter to the cloud and back again, the actual height of the cloud (from the transmitter) is one-half of the total distance. The calculation is expressed as:
h = ct/2
where:
h = Height of Cloud
c = Speed of Light (9.8356 x 10^8 feet/second)
t = Time from Transmission to Reception
For example: A Return Pulse detected 24.4 microseconds (24.4 x 10^-6 seconds) after transmission shows a cloud at 12,000 feet.
Precipitation Identification Sensor
 The Precipitation Identification Sensor detects precipitation type and measures precipitation intensity. The Precipitation Identification Sensor uses weather-particle-induced optical scintillation of an infrared emitter diode (IRED) system to identify precipitation state and type and measure precipitation intensity. The Precipitation Identification Sensor reports three precipitation conditions to the Acquisition Control Unit (ACU). These are rain, snow, and precipitation undetermined. The Precipitation Identification Sensor also reports the intensity of the precipitation as light, moderate, or heavy. The ASOS automatically defines fog and haze conditions based on the current visibility parameter.
The Precipitation Identification Sensor detects precipitation type and measures precipitation intensity. The Precipitation Identification Sensor uses weather-particle-induced optical scintillation of an infrared emitter diode (IRED) system to identify precipitation state and type and measure precipitation intensity. The Precipitation Identification Sensor reports three precipitation conditions to the Acquisition Control Unit (ACU). These are rain, snow, and precipitation undetermined. The Precipitation Identification Sensor also reports the intensity of the precipitation as light, moderate, or heavy. The ASOS automatically defines fog and haze conditions based on the current visibility parameter.
Visibility & Day/Night Sensor
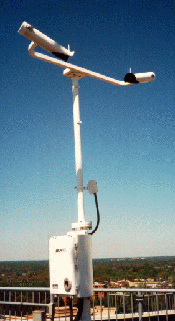 The Visibility and Day/Night Sensor, (the Visibility Sensor), measures visibility(see below). It provides the means to calculate the current visibility level automatically and show current day/night conditions. The Visibility Sensor measures ambient visibility using the Forward Scatter Technique. This technique transmits a flash of xenon(see below) light through a section of the atmosphere. The atmosphere scatters the light. The scattered light level is measured to detect the loss of light. An Extinction Coefficient is calculated from how much light is received from the scattered xenon flash lamp light source. This coefficient is then translated into a value of visibility.
The Visibility and Day/Night Sensor, (the Visibility Sensor), measures visibility(see below). It provides the means to calculate the current visibility level automatically and show current day/night conditions. The Visibility Sensor measures ambient visibility using the Forward Scatter Technique. This technique transmits a flash of xenon(see below) light through a section of the atmosphere. The atmosphere scatters the light. The scattered light level is measured to detect the loss of light. An Extinction Coefficient is calculated from how much light is received from the scattered xenon flash lamp light source. This coefficient is then translated into a value of visibility.
The Visibility Sensor also computes a day or night indication as derived from an ambient light sensor. Both the Extinction Coefficient and day/night information are sent to and used by the Data Collection Package (DCP).
Visibility is a measure of the opacity of the atmosphere and is expressed in terms of the horizontal distance at which a person can see and identify specified objects. Xenon is a colorless, odorless, highly unreactive gas that is found in very small quantities in the atmosphere. Its atomic number is 54.
Wind Sensor
 The Wind Sensor measures current wind speed and direction and computes 5-second average of these measurements. Wind speed is measured by a rotating three-cup device that drives a photointerrupter device. Wind direction is measured with a direction vane assembly coupled to a precision potentiometer. Wind measurement data is sent to the Data Collection Package (DCP).
The Wind Sensor measures current wind speed and direction and computes 5-second average of these measurements. Wind speed is measured by a rotating three-cup device that drives a photointerrupter device. Wind direction is measured with a direction vane assembly coupled to a precision potentiometer. Wind measurement data is sent to the Data Collection Package (DCP).
The Wind Sensor consists of four major components: Wind Speed Sensor, Wind Direction Sensor, Crossarm Support, and Wind Sensor Electronics Enclosure. The Wind Speed Sensor is made up of a wind speed transducer and a cup assembly. Similarly, the Wind Direction Sensor comprises a wind direction transducer and a vane assembly. The Wind Speed Sensor, Wind Direction Sensor, and Crossarm Support are all mounted on a tipping tower to allow them to operate free from ground obstructions while still being easy to maintain.
The Wind Sensor Electronics Enclosure provides electrical power to the wind sensor and a communications data link between the sensor and the DCP. The wind sensor electronics enclosure is mounted on the sensor tower support.
By the 1940’s military aircraft were being used to make high altitude observations. In the 1960’s the National Weather Service got its first weather satellite and forecasting major weather events became easier. Despite these advances the technology for measuring weather on the ground had changed little.
In 1990 the National Weather Service began updating its ground weather stations with Automated Surface Observing Systems (ASOS). By the mid 1990’s there were 1700 ASOS units operating in the United States. All surface weather information is now collected by ASOS systems but most data is still double-checked using the traditional equipment and all snow measurements are still done manually using the traditional methods.
This Microsoft Access file provides access to climate data (precipitation and temperature) for all stations in Idaho operated by the Northwest Regional Climate Center from 1952 to 1999..
