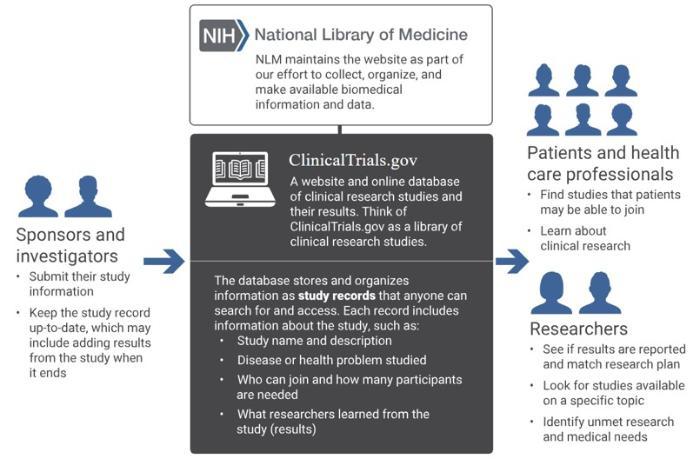
ClinicalTrials.gov
ClinicalTrials.gov is a publicly available website and searchable online database of clinical research studies and information about their results. The purpose of ClinicalTrials.gov is to provide information about clinical research studies to the public, researchers, and health care professionals.
(image source: Clinical Trials.gov)
The National Institutes of Health (NIH) defines a research study as a clinical trial if all of the following conditions are true.
- The study involves human participants.
- Participants are assigned to an intervention.
- The study is designed to evaluate the effect of the intervention on the participants.
- The effect being evaluated is a health-related or behavioral outcome.
Contact the ISU ClinicalTrials.gov Administrator at 208-282-2618 if you need assistance determining whether a research study is a clinical trial.
An intervention is a specific action or treatment implemented as part of a study or experiment. Interventions include medication, therapy, biologics, medical devices, and behavior modification.
All clinical trials should be registered at ClinicalTrials.gov regardless of the funding source.
Registering clinical trials is required by law with few exceptions. Links to relevant regulations may be found in the Additional Resources section below. In addition, many publishers such as the International Committee of Medical Journal Editors (ICMJE) require registration as a condition of publication.
In general, PIs should register a study at ClinicalTrials.gov as soon as they are confident the clinical trial will take place. The Food and Drug Administration Amendments Act requires registration of clinical trials within 21 days of enrollment of the first participant. In addition, the International Committee of Medical Journal Editors (ICMJE) requires registration prior to the enrollment of the first participant.
The study's Principal Investigator (PI) is the responsible party. Responsibilities include registering the study, submitting results, updating results as needed, responding to comments from ClinicalTrials.gov reviewers, and closing the study at the conclusion of the clinical trial.
The Research Integrity and Compliance Department within the Office for Research administers ClinicalTrials.gov for ISU. Contact the ISU ClinicalTrials.gov Administrator at 208-282-2618 if you need to register, need assistance with the system, or have questions.
You may request training by contacting the ISU ClinicalTrials.gov Administrator at 208-282-2618. The answers to many questions about the system may be found at ClinicalTrials.gov FAQ.
Questions may be directed to ClinicalTrials.gov directly via email at ClinicalTrials.gov.
ClinicalTrials.gov Frequently Asked Questions
42 CFR 11 - Clinical Trials Registration and Results Submission
45 CFR 46 - Protection of Human Subjects (relevant sections are 46.102(b) and 46.116(h))
